The Integrated Research Facility at Fort Detrick (IRF‑Frederick) provides unique expertise and capabilities to address scientific questions. The IRF‑Frederick has an established yet adaptable process that sets the research agenda through the identification and review of collaborative projects that fit the IRF-Frederick mission. (For more about the mission, go to Integrated Research Facility at Fort Detrick).
The IRF-Frederick facilitates high-quality research by managing and conducting studies that use the unique capabilities of the facility. The IRF-Frederick provides flexibility and access to resources, giving collaborators a unique opportunity to choose how much support they need from the IRF-Frederick and, conversely, how much they will contribute to the studies. The relationship between the IRF-Frederick and each collaborating organization is based on the scope, relevance, and attributes of the specific research project.
Portfolio Development Process
Portfolio development begins with a concept, fostered by communication among scientists at the IRF‑Frederick and NIH intramural or extramural investigators (including those from government, academia, industry, non-profits, and other partner organizations). A collaborative research proposal is developed and submitted based on the following two categories:
- Research projects with collaborators that have approved NIH-funded grants—Proposals for research within this category are evaluated by the IRF-Frederick and, if approved, do not need further review.
- All other research projects—Proposals for research within this category are reviewed by both a committee within the IRF‑Frederick and the NIAID Scientific Steering Committee. The committees make recommendations based on mission relevance and scientific value to ensure fair and equitable allocation of IRF-Frederick resources.
Once a proposal is approved, the IRF-Frederick commences with project prioritization, resource allocation, and operational planning.
This could involve implementing a collaborative agreement, such as:
- Memorandum of understanding (MOU)
- Research collaborative agreement (RCA)
- Interagency agreement (IAA)
- Material transfer agreement (MTA)
- Non-disclosure agreement (NDA)
The IRF-Frederick is a national resource and, as such, does not charge collaborators for research project activities conducted at the facility. However, purchase of animals and specialty reagent costs are covered by the collaborators.
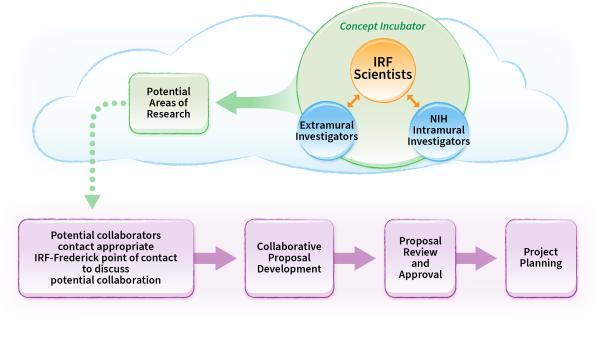
The IRF-Frederick has an established process that identifies and reviews suitable projects, which sets the research agenda.
How to Get Started
Each of the IRF-Frederick resources (listed below) has a point of contact who can help researchers with requesting collaboration and developing a research project. For specific collaboration guidance in one of those areas, go to the appropriate resource page (under Resources for Researchers).
For general information about research collaboration, contact the IRF-Frederick directly (NIAIDIRFFrederick@niaid.nih.gov).
Learn more information about collaborations.
Resources for Researchers
The IRF-Frederick has the following capabilities and collaborative research support opportunities available to internal (NIAID) and extramural researchers. Contact information is included, so researchers can reach out to someone to guide them through the process.
- Aerobiology
- AIDS Imaging Research
- Animal Models of Disease
- Artificial Intelligence
- Clinical Studies Support
- Core Services
- Drug Screening
- Electron Microscopy (EM)
- Genomics
- Imaging Sciences
- Immunology
- Pathology and Histology
- Virology
Perform a keyword search of opportunities offered by the IRF-Frederick.

