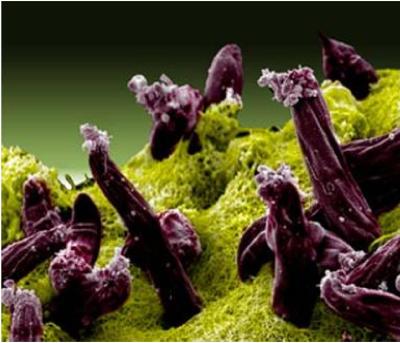
Scanning electron micrograph of Plasmodium gallinaceum invading mosquito midgut.
NIAID encourages Small Business applications to better understand, treat, and ultimately prevent infectious diseases caused by infectious agents. The Division of Microbiology and Infectious Diseases (DMID) supports a broad spectrum of research—from basic molecular structure, microbial physiology, and pathogenesis to the development of new and improved vaccines and therapeutics. DMID also supports medical diagnostics research to improve the quality of patient assessment and care, and result in implementing appropriate therapeutic or preventive measures. DMID does not support research directed at decontamination, disinfection, or the development of environmentally oriented detectors, whose primary purpose is identifying specific agents in the environment.
Listed below are the general research areas of interest to DMID and NIAID’s Small Business Program. Examples of high-priority research topics include but are not limited to antimicrobial resistance, coronavirus, influenza, malaria, and tuberculosis.
If you have questions about the application process, research topics or areas of interest, or wish to be directed to an appropriate subject matter expert within DMID, please contact Barbara Mulach or Alyssa Werner in DMID’s Office of Scientific Coordination and Program Operations.
Areas of Interest
- Identification and qualification of infectious disease-related biomarkers, including:
- Biomarkers to predict susceptibility to infection and/or diagnose an infectious disease.
- Biomarkers to predict or monitor a subject’s response to therapeutics or vaccinations.
- Biomarkers from natural history studies that could be used to assess disease progression in acute and chronic diseases.
- Development of rapid, highly sensitive and specific clinical diagnostics that are easy to use, cost-effective, and can diagnose individuals infected with pathogens or individuals who have been exposed to toxins.
- Discovery and development of vaccines or other immunoprophylaxis tools for infectious diseases.
- Development of vaccine enhancement and formulation technologies to improve functionality and manufacturability, generate accelerated immune responses (e.g., more rapid schedules or reduced number of immunizations), increase ease of administration and distribution (e.g., self-administration), and improve product stability to minimize cold chain requirements.
- Discovery and development of therapeutics for infectious diseases, including immunotherapeutics or other biologicals, for treatment of infectious diseases.
- Development of technologies or approaches that address arthropod vector monitoring, management, and control to prevent transmission of vector-borne pathogens to humans.

