Genetic Engineering Section
Bernard Moss, M.D., Ph.D.
Chief, Genetic Engineering Section
NIH Distinguished Investigator

Major Areas of Research
- Replication of poxviruses
- Viral immune defense proteins
- Recombinant vaccines
Program Description
The goals are to determine the mechanisms used by viruses to infect cells, express and replicate their genomes, assemble infectious particles, and evade the host immune response. Basic information obtained from these studies is used to design antiviral agents and live and subunit recombinant vaccines. In addition, viruses are engineered as expression vectors for biotechnology.
Poxviruses have provided unique opportunities to combine molecular, genetic, microscopic, and immunologic approaches to achieve many of the above goals. Recent studies have also been directed to the characterization and testing of candidate vaccines for human and simian immunodeficiency viruses.
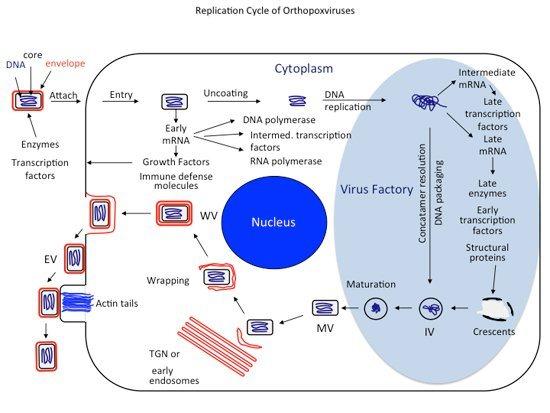
Replicaton Cycle of Orthopoxviruses
Virions—containing a double-stranded DNA genome, enzymes, and transcription factors—attach to cells and fuse with the cell membrane, releasing cores into the cytoplasm. The cores synthesize early mRNAs that are translated into a variety of proteins, including growth factors, immune defense molecules, enzymes, and factors for DNA replication and intermediate transcription. Uncoating occurs, and the DNA is replicated to form concatemeric molecules. Intermediate genes in the progeny DNA are transcribed, and the mRNAs are translated to form late transcription factors. The late genes are transcribed and the mRNAs are translated to form virion structural proteins, enzymes, and early transcription factors. Assembly begins with the formation of discrete membrane structures. The concatemeric DNA intermediates are resolved into unit genomes and packaged in immature virions. Maturation proceeds to the formation of infectious intracellular mature virions (MVs). The virions are wrapped by modified trans-Golgi and endosomal cisternae, and the wrapped virions (WVs) are transported to the periphery of the cell via microtubules. Fusion of the wrapped virions with the plasma membrane results in release of extracellular enveloped virions (EVs).
Biography
Education
Ph.D., Massachusetts Institute of Technology
M.D., New York University School of Medicine
Dr. Moss received his M.D. from the New York University School of Medicine, interned at the Children's Hospital Medical Center (Boston), and then earned a Ph.D. in biochemistry from the Massachusetts Institute of Technology. He became interested in viruses after joining NIH and is well known for studies on the cap structure of mRNAs, regulation of gene expression, replication cycle of poxviruses, virus defense molecules, and development and application of virus vectors. Dr. Moss has received numerous awards and prizes, including the Dickson Prize for Medical Research, the Invitrogen Eukaryotic Expression Award, the ICN International Prize in Virology, the Taylor International Prize in Medicine, the Bristol-Myers Squibb Award for Distinguished Achievement in Infectious Disease Research, the International Poxvirus, Asfarvirus and Iridovirus Lifetime Achievement Award and the ASM Lifetime Achievement Award. He was elected to the National Academy of Sciences, American Academy of Microbiology, Fellow of the American Association for the Advancement of Science, and president of the American Society for Virology. Dr. Moss is currently on the editorial boards of the Proceedings of the National Academy of Sciences, the Journal of Virology, and the NIH Catalyst. He is an adjunct professor at the University of Maryland. Special Interest Groups: Virology, Immunology, Transcription
Selected Publications
Senkevich TG, Yutin N, Wolf YI, Koonin EV, Moss B. Ancient Gene Capture and Recent Gene Loss Shape the Evolution of Orthopoxvirus-Host Interaction Genes. mBio. 2021 Aug 31;12(4):e0149521.
Liu R, Americo JL, Cotter CA, Earl PL, Erez N, Peng C, Moss B. One or two injections of MVA-vectored vaccine shields hACE2 transgenic mice from SARS-CoV-2 upper and lower respiratory tract infection. Proc Natl Acad Sci U S A. 2021 Mar 23;118(12):e2026785118.
Moss B. Investigating Viruses During the Transformation of Molecular Biology: Part II. Annu Rev Virol. 2020 Sep 29;7(1):15-36.
Peng C, Wyatt LS, Glushakow-Smith SG, Lal-Nag M, Weisberg AS, Moss B. Zinc-finger antiviral protein (ZAP) is a restriction factor for replication of modified vaccinia virus Ankara (MVA) in human cells. PLoS Pathog. 2020 Aug 31;16(8):e1008845.
Earl PL, Americo JL, Moss B. Natural killer cells expanded in vivo or ex vivo with IL-15 overcomes the inherent susceptibility of CAST mice to lethal infection with orthopoxviruses. PLoS Pathog. 2020 Apr 22;16(4):e1008505.
Liu R, Olano LR, Mirzakhanyan Y, Gershon PD, Moss B. Vaccinia Virus Ankyrin-Repeat/F-Box Protein Targets Interferon-Induced IFITs for Proteasomal Degradation. Cell Rep. 2019 Oct 22;29(4):816-828.e6.
Videos
Visualization of movement of fluorescently-labeled wrapped virions (IEV) from factory region to periphery of the cell
At 12 hours after infection, images were collected at 1 frame/sec for a period of 110 sec.
From Ward BM, Moss B. Visualization of intracellular movement of vaccinia virus virions containing a green fluorescent protein-B5R membrane protein chimera. J Virol. 2001 May; 75(10):4802-13.
Visualization of fluorescently labeled actin tails tipped by vaccinia virions
At 12 h after infection, images were collected at 1 frame/sec.
From Ward BM, Moss B. Vaccinia virus intracellular movement is associated with microtubules and independent of actin tails. J Virol. 2001 Dec;75(23):11651-63.
Rotating 3D model of an A-Type inclusion (ATI) body with embedded with vaccinia virions in a HeLa cell
The IMARIS Surfaces feature was used to define the ATI as a region of interest, which is depicted visually in the model as a red shell. The virions fluorescing green are within and outside the ATI shell.
From Howard A, Moss B. Formation of orthopoxvirus cytoplasmic A-type inclusion bodies and embedding of virions are dynamic processes requiring microtubules. J Virol. 2012 May;86(10):5905-14.
Time lapse video of ATI coalescence
Infected cells were imaged as Z-stacks at 20 minute intervals for 4 hours. IMARIS Surfaces was used to track an individual ATI (dragontail) and measure virion embedment as fluorescence within the defined surface over time. ATIs, red; Virions, green.
From Howard A, Moss B. Formation of orthopoxvirus cytoplasmic A-type inclusion bodies and embedding of virions are dynamic processes requiring microtubules. J Virol. 2012 May;86(10):5905-14.
Saltatory movement of virions to ATIs
Video shows a virion tracked using the IMARIS Spots feature move to and associate with an ATI.
From Howard A, Moss B. Formation of orthopoxvirus cytoplasmic A-type inclusion bodies and embedding of virions are dynamic processes requiring microtubules. J Virol. 2012 May;86(10):5905-14.
Research Group
The research group investigates the replication of poxviruses, viral immune defense proteins and recombinant vaccines against other viral pathogens including monkeypox virus, coronaviruses, and immunodeficiency viruses.


