Viral Immunity and Pathogenesis Unit
Heather Hickman, Ph.D.
Chief, Viral Immunity and Pathogenesis Unit

Major Areas of Research
- Biology of tissue-resident lymphocytes at initial sites of viral infection
- Role of the lymph node in shaping antiviral immunity
- T cell-mediated clearance of established viral infections
- Viruses studied: poxviruses, influenza, Zika, Chikungunya, SARS-CoV-2
Program Description
Our laboratory’s primary goal is to gain fundamental insights into the biology of antiviral effector cells in both peripheral and lymphoid tissues. The Viral Immunity and Pathogenesis Unit (VIPU) seeks to better understand the function and antiviral mechanisms employed by these lymphocytes in infected tissues as the immune response occurs in real time in vivo.
To understand quantitative phenotypic, mechanistic and spatial components of antiviral immunity, the VIPU utilizes traditional ex vivo immunologic and virologic techniques along with multiplex immunohistochemistry of infected tissue sections and intravital multiphoton microscopy (MPM) of living mice, an imaging modality allowing the direct visualization of virus-infected cells and immune effectors as they move and interact over time in vivo. Using this multi-tiered approach, the VIPU examines three distinct but complementary themes in antiviral immunity:
- Biology of tissue-resident lymphocytes at initial sites of viral infection
- Role of the lymph node in shaping antiviral immunity
- T cell-mediated clearance of established viral infections
Rather than focus on a single viral pathogen, the VIPU investigates immunity to a wide range of viruses, from large DNA viruses, such as vaccinia (VACV) and herpes simplex virus (HSV), to small RNA viruses including Zika (ZIKV) and influenza virus (IAV). Using disparate murine viral infection models, the VIPU aims to discover broad, shared features that contribute to protective antiviral immunity while also discerning virus-specific aspects of immune control.
Current studies in the laboratory involve better understanding group I innate lymphoid cell protection of mucosal tissues; investigating the role of the lymphatic vasculature and lymph node anatomy in restricting or promoting viral infection; and understanding the different mechanisms that allow cytotoxic T cells to locate and eliminate infected cells in the complex tissue environment.
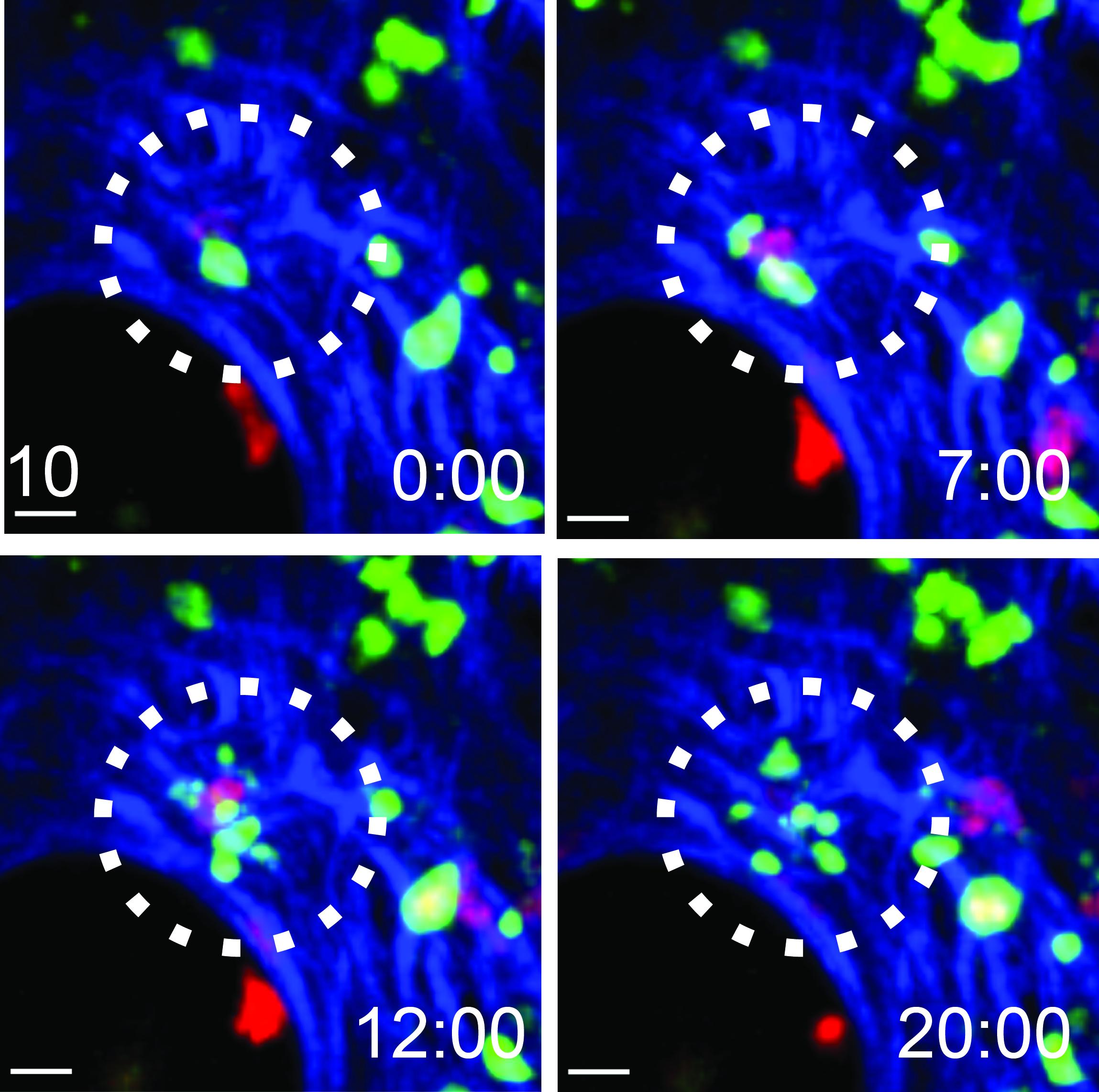
Image 1. A cytotoxic T cell (red) kills a vaccinia virus-infected cell (green) in the skin (highlighted with a circle). Collagen of the dermis is shown in blue. Time in min:sec is indicated in the lower right corner. Credit: NIAID
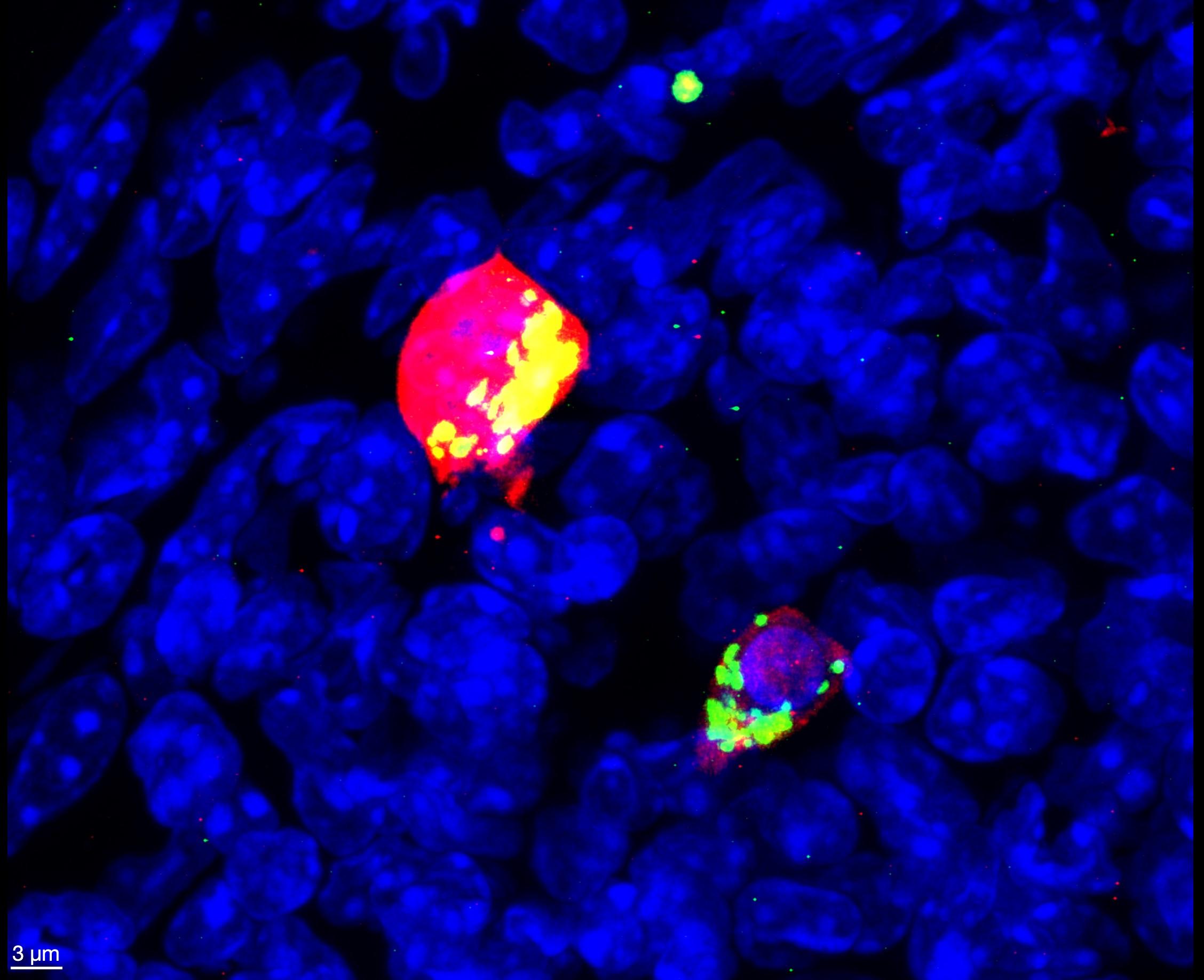
Image 2. The location and distribution of mitochondria in cytotoxic T cells changes dynamically during infection. Shown is a cytotoxic T cell (red) with a fluorescent protein (Dendra, green and red) fused to the mitochondria. Nuclei are stained with DAPI, blue. Credit: NIAID
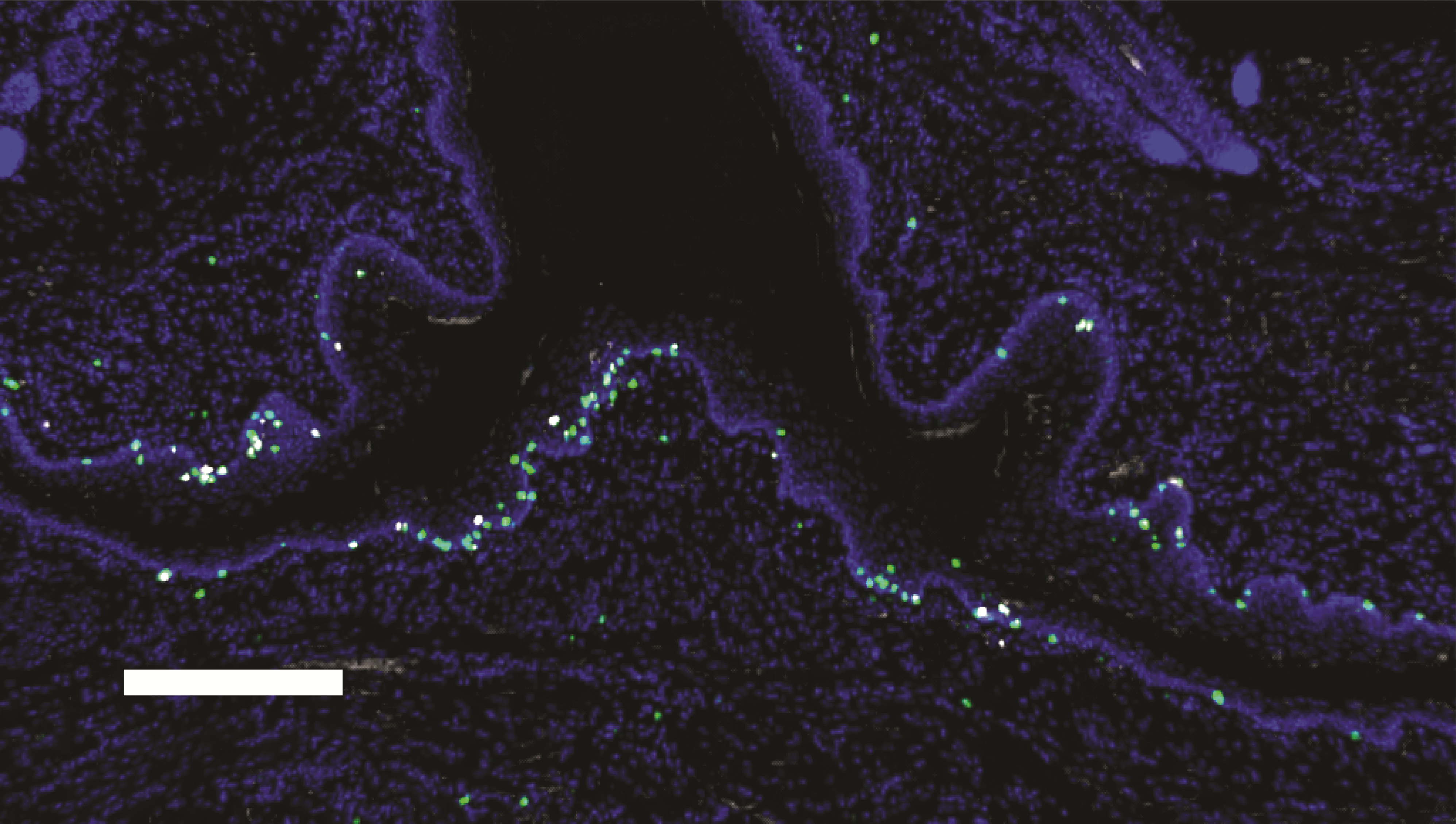
Image 3. Cytotoxic group I innate lymphoid cells (ILC1s, green) populate the murine oral mucosa. The uninfected oral mucosa is shown stained for nuclei (DAPI, blue) and granzyme C (white), a marker identifying mature, potentially cytotoxic ILC1s. Credit: NIAID
Biography
Education
Ph.D., University of Oklahoma
Dr. Heather Hickman received her Ph.D. in Microbiology and Immunology from the University of Oklahoma, where she investigated the presentation of virus-derived peptides by major histocompatibility (MHC) class I molecules. Dr. Hickman first joined the NIH as a postdoctoral fellow in the Laboratory of Diseases, NIAID, to study antiviral immunity under the mentorship of Dr. Jonathan Yewdell.
Selected Publications
Vrba SM, Hickman HD. Imaging viral infection in vivo to gain unique perspectives on cellular antiviral immunity. Immunol Rev. 2022 Mar;306(1):200-217.
Lujan RA, Vrba SM, Hickman HD. Antiviral Activities of Group I Innate Lymphoid Cells. J Mol Biol. 2022 Mar 30;434(6):167266.
Carpentier KS, Sheridan RM, Lucas CJ, Davenport BJ, Li FS, Lucas ED, McCarthy MK, Reynoso GV, May NA, Tamburini BAJ, Hesselberth JR, Hickman HD, Morrison TE. MARCO+lymphatic endothelial cells sequester arthritogenic alphaviruses to limit viremia and viral dissemination. EMBO J. 2021 Nov 15;40(22):e108966.
Shannon JP, Vrba SM, Reynoso GV, Wynne-Jones E, Kamenyeva O, Malo CS, Cherry CR, McManus DT, Hickman HD. Group 1 innate lymphoid-cell-derived interferon-γ maintains anti-viral vigilance in the mucosal epithelium. Immunity. 2021 Feb 9;54(2):276-290.e5.
Reynoso GV, Weisberg AS, Shannon JP, McManus DT, Shores L, Americo JL, Stan RV, Yewdell JW, Hickman HD. Lymph node conduits transport virions for rapid T cell activation. Nat Immunol. 2019 May;20(5):602-612.
Cush SS, Reynoso GV, Kamenyeva O, Bennink JR, Yewdell JW, Hickman HD. Locally Produced IL-10 Limits Cutaneous Vaccinia Virus Spread. PLoS Pathog. 2016 Mar 18;12(3):e1005493.
Research Group
The Viral Immunity and Pathogenesis Unit aims to make fundamental discoveries about the mechanisms underlying effective antiviral immune responses.

