The major focus of the Cellular Immunology Section over the past 25 years has been furthering our understanding of the function of T regulatory cells (Tregs) that express the transcription factor Foxp3. Our group was one of the first in the world to realize the importance of Treg and we performed many of the initial studies that described their phenotype and function. The study of Tregs is now one of the most active areas of research in basic and clinical immunology and the therapeutic use of Treg is now in the clinic. It was originally assumed that Treg were a dedicated lineage of cells that developed only from thymic precursors, but more recent studies have clearly documented that Foxp3+ T cells also develop from conventional T cells (Tconv) in extra-thymic peripheral sites in vivo, and these are termed peripherally induced Tregs (pTregs). Treg can also be generated in culture in the presence of transforming growth factor-b1 (TGF-b1) and are termed induced Tregs (iTreg). The relative importance of tTreg and pTreg is unknown.
Although most of our studies deal with Tregs in mouse models, over the past 15 years we have also carried out studies on human Tregs (hTregs) derived from normal donors. Our ongoing studies are described below and have been divided up into five major projects with significant overlap between the projects. We also intentionally validate new and novel findings that we learn in one species with similar studies in another species, as there appears to be conservation of many aspects of Treg function across the species.
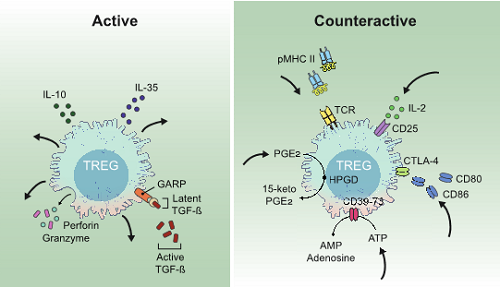
Two fundamentally different suppressor modes characterize Treg suppressor mechanisms. In the “active” mode, Treg cells secrete immunoinhibitory molecules that exert their effects on other cell types. In contrast, in the “counteractive” mode Treg cells are actively engaged in removing vital components from other cells types including antigen, costimulatory molecules, cytokines, and inflammatory signals thereby decreasing the activation of T effector cells. B. Akkaya and E.M. Shevach, Cellular immunology 2020.
Ethan M. Shevach, M.D.
Chief, Cellular Immunology Section
Education:
M.D., 1967, Boston University

Angela Thornton, Ph.D.
Staff Scientist
Education:
Ph.D., Microbiology and Immunology, The George Washington University
B.A., Biology, University of Virginia
Pat Korty, M.S.
Microbiologist
Education:
M.S., Animal Science, The University of Maryland
Abir Panda, Ph.D.
Research Fellow (VP)
Education:
Ph.D., Biotechnology, Bose Institute, India
Xuan Xie, Ph.D.
Visiting Postdoctoral Fellow
Education:
Ph.D., Biological Sciences, Tokyo Institute of Technology, Japan
Yong-Hee Kim, M.D., Ph.D.
Visiting Postdoctoral Fellow
Education:
M.D., Ph.D., Immunology, Seoul National University College of Medicine, South Korea
Mohammad Nizam Mansoori, Ph.D.
Visiting Postdoctoral Fellow
Education:
Ph.D., Osteoimmunology, Council of Scientific and Industrial Research – Central Drug Research Institute, India
Melissa Blain, M.S.
Biologist
Education:
M.S., Medical Biology, C.W. Post Campus of Long Island University
B.S., Biochemical Pharmacology, University at Buffalo
Sruthi Chempati, M.S.
Biologist
Education:
M.S., Biological Sciences, Texas A&M University
B.S., Biotechnology, Jawaharlal Nehru Technological University
Madalyn Jones, B.S.
Postbaccalaureate Fellow
Education:
B.S., Biomedical Sciences, Troy University
Neil Yang, B.S.
Postbaccalaureate Fellow
Education:
B.S., Biology, Case Western Reserve University
Soha Kazmi, B.S.
Postbaccalaureate Fellow
Education:
B.S., Biology and Chemistry, University of Florida
Sooho Myoung, B.S.
Postbaccalaureate Fellow
Education:
B.S., Statistics, The University of North Carolina at Chapel Hill

Cellular Immunology Section, 2023. Front row (left to right): Xuan Xie, Visiting Postdoctoral Fellow, Soha Kazmi, Postbaccalaureate Fellow, Ethan Shevach, Principal Investigator, Pat Korty, Microbiologist, Madalyn Jones, Postbaccalaureate Fellow, Angela Thornton, Staff Scientist. Back Row (left to right): Abir Panda, Visiting Postdoctoral Fellow, Nizam Mansoori, Visiting Postdoctoral Fellow, Neil Yang, Postbaccalaureate Fellow, Yong-Hee Kim, Visiting Postdoctoral Fellow, Melissa Blain, Biologist, Suveena Sharma (alumnus), Biologist. Top Left Corner (left to right): Sruthi Chempati, Biologist, Sooho Myoung, Postbaccalaureate Fellow.




























































