Molecular Defenses Section
Established in 1990
Thomas Leto, Ph.D.
Chief, Molecular Defenses Section

Major Areas of Research
- NOX/DUOX family NADPH oxidases, structure and function
- NOX-based innate immune mechanisms in phagocytic cells and in respiratory and gastrointestinal tracts
- Roles of reactive oxygen and NOX enzymes in health and disease (host defense, inflammation, cellular signaling, innate and adaptive immunity)
- Roles of NOX enzymes in tumor microenvironment signaling, cancer progression, and survival
Program Description
This program explores innate immune, pro-inflammatory, and signaling functions of NOX family NADPH oxidases. Current research focuses on non-phagocytic NADPH oxidases (NOX1, NOX4, DUOX1, DUOX2) expressed primarily in epithelial cells, as well as NOX2 in hematopoietic cells. Reactive oxygen species (ROS) production by NOX enzymes relays redox signals in responses to cytokines, chemokines, growth factors, hormones, and danger- and pathogen-associated molecular patterns (DAMPs and PAMPs). In addition to serving direct microbicidal roles, NOX-derived ROS regulate cell migration, proliferation, differentiation, senescence, apoptosis, tumor invasiveness and metastasis. We are exploring functions of several NOX family NADPH oxidase components in two general areas: 1) studies on genetic variants of NOX and DUOX components associated with innate antimicrobial defense and barrier function defects or inflammatory disease, 2) studies on roles of NADPH oxidases in cancer progression.
Our interests in rare NOX and DUOX genetic variants in patients with innate immune defects and inflammatory disease syndromes originated with identification of NOX2 component (p47phox and p67phox) defects in patients with chronic granulomatous disease (CGD). As whole exome sequencing (WES) data has become widely available from patients of our clinical collaborators, we explore mechanistic consequences of oxidase defects using heterologous oxidase expression systems. In studies on several loss-of-function (LoF) DUOXA1 and DUOX1 variants linked to pulmonary and disseminated fungal infection we reconstituted signaling pathways linking DUOX1 activation with Dectin1 fungal pathogen receptor detection and signaling. We showed rare NOX1 variants detected in patients with inflammatory bowel disease exhibit low NOX1 activity and support lower NOX1-dependent cell migration in our reconstituted model of colon epithelial wound closure and barrier function (NHGRI collab.). We also characterized several partially functional CYBA, CYBB, and NCF1 variants linked to enhanced susceptibility to microbial infection and inflammation atypical of CGD. Lastly, we are studying RAC2 variants associated with neutrophil dysfunction, lymphopenia and immunodeficiencies.
Our interests in NOX function in cancer originated from work in lung, breast, liver, and pancreatic tumor cell lines showing NOX4 is induced in a TGF-beta- and SMAD3-dependent manner in tumors bearing TP53 hot spot mutations; this involves enhanced histone acetylation once mutant p53 binds the NOX4 promoter, leading to increased NOX4-dependent cell migration. We have undertaken an in depth bioinformatic analysis of primary human tumors of 23 cancer types in The Cancer Genome Atlas (TCGA) and validated our in vitro observations on the roles of NOX4 in promoting programs of cancer progression in a variety of tumor types with p53 hotspot mutations. We found high NOX4 tumor expression correlates with poor survival in patients with mutant TP53 tumors, whereas survival was better in patients with high NOX4 tumor levels in wild type TP53 tumors. We confirmed NOX4 expression correlates with genetic programs supporting tumor cell migration, invasiveness, and angiogenesis, specifically in tumors where TP53 is mutated regardless of tumor tissue origin. NOX4 expression correlates positively with gene programs associated with cell proliferation and negatively with programs of cell apoptosis in tumors with mutant TP53, while the opposite was observed in tumors with wild-type TP53. Thus, p53 mutations switch NOX4 from acting as a protective oxidase predicting a good prognosis to one with deleterious effects on cancer progression and clinical outcome in late-stage cancers.
Complementary studies are examining Nox expression patterns in a mouse model of pancreatic ductal adenocarcinoma (PDAC) caused by pancreatic-targeted mutations in TP53 and K-ras. These tumors show a 10-15-fold enhancement of Nox4 and Nox2 levels relative to normal pancreatic tissue, consistent with our TCGA informatics analysis of human PDAC datasets. High Nox4 expression was detected in ductal epithelial tumor cells as well as in adjacent fibrotic stomal tissue, while highest Nox2 (Cybb) expression was detected in tumor associated macrophages. Current experiments are exploring whether the absence of functional Nox4 affects tumor cell escape from primary tumor sites or the access or immunophenotype of inflammatory cells within the tumor microenvironment. Related cell culture experiments are exploring NOX-dependent chemotactic and inflammatory signaling between macrophages and tumor cells as a model of the tumor microenvironment.
Biography
Education
Ph.D., Biochemistry, University of Virginia
Dr. Leto received his Ph.D. in biochemistry from the University of Virginia for studies on mechanisms of cell membrane assembly. He followed this work with postdoctoral studies at Yale University on membrane cytoskeleton interactions. Dr. Leto joined NIAID in 1988 and became a senior investigator in the Laboratory of Host Defenses in 1996.
Special Interest Groups: Cell Biology, Structural Biology, Free Radical/Oxygen Club
Selected Publications
- Donko A, Kuhns DB, Cousin MA, Smith MJ, Sacco KA, Klee EW, Joshi AY, Gavrilova RH, Holland SM, Leto TL, Abraham RS. Interpretation of Dihydrorhodamine-1,2,3 Flow Cytometry in Chronic Granulomatous Disease: an Atypical Exemplar. J Clin Immunol. 2022 Mar 28.
- Ma WF, Boudreau HE, Leto TL. Pan-Cancer Analysis Shows TP53 Mutations Modulate the Association of NOX4 with Genetic Programs of Cancer Progression and Clinical Outcome. Antioxidants (Basel). 2021 Feb 4;10(2):235.
- Hsu AP, Donkó A, Arrington ME, Swamydas M, Fink D, Das A, Escobedo O, Bonagura V, Szabolcs P, Steinberg HN, Bergerson J, Skoskiewicz A, Makhija M, Davis J, Foruraghi L, Palmer C, Fuleihan RL, Church JA, Bhandoola A, Lionakis MS, Campbell S, Leto TL, Kuhns DB, Holland SM. Dominant activating RAC2 mutation with lymphopenia, immunodeficiency, and cytoskeletal defects. Blood. 2019 May
- Korzeniowska A, Donkó ÁP, Morand S, Leto TL. Functional Characterization of DUOX Enzymes in Reconstituted Cell Models. Methods Mol Biol. 2019;1982:173-190.
- Boudreau HE, Leto TL. Model Systems to Investigate NOX-Dependent Cell Migration and Invasiveness. Methods Mol Biol. 2019;1982:473-485.
- Boudreau HE, Ma WF, Korzeniowska A, Park JJ, Bhagwat MA, Leto TL. Histone modifications affect differential regulation of TGFβ- induced NADPH oxidase 4 (NOX4) by wild-type and mutant p53. Oncotarget. 2017 Jul 4;8(27):44379-44397.
Images
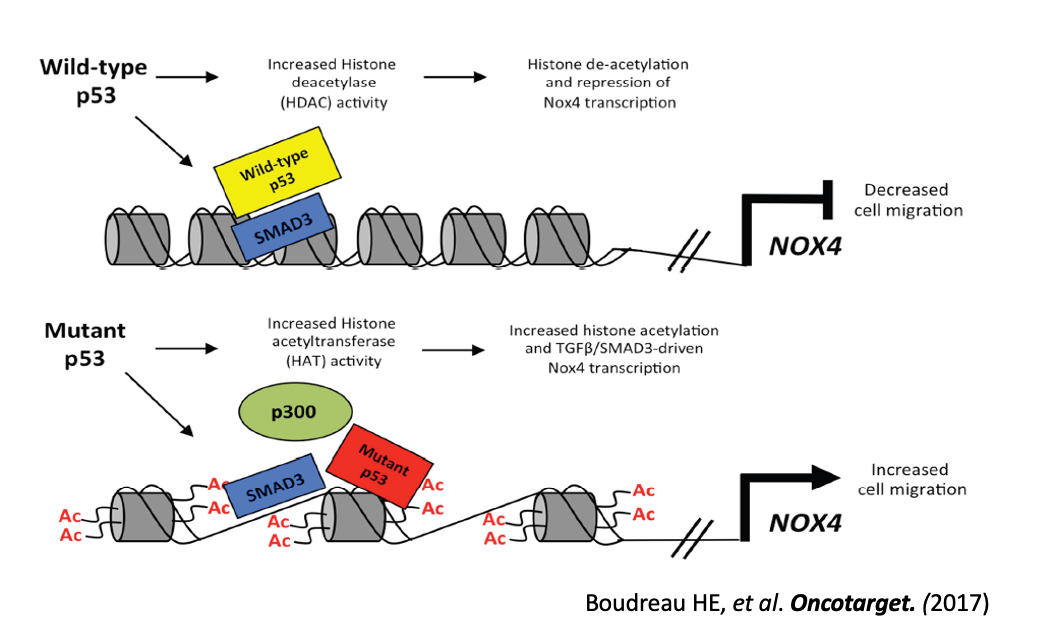
Model of the divergent roles of wild-type and mutant p53 regulating NOX4 expression and cell migration through histone modifications.
Research Group
MDS explores various aspects of NADPH oxidase (NOX/DUOX) function in the contexts of infectious and inflammatory diseases and cancer progression.

