Medical interventions take years of scientific research and are often achieved through multiple partnerships and collaborations. The Division of Microbiology and Infectious Diseases (DMID) supports research and provides resources for all stages of product development, partnering with public and private institutions to move advances through the product development pipeline. The results are research discoveries that are transforming the prevention, diagnosis, and treatment of infectious diseases. The DMID scientific success showcase highlights just a few of these achievements.
Fighting Fire With Fire: Healthy Bacteria May Help Combat Infection
December 13, 2024An NIAID-supported research company called Osel has developed a novel product called LACTIN-V that consists of a selected strain of Lactobacillus crispatus, one of the bacteria that protect the vagina from invading pathogens, that has been freeze-dried and formulated as a powder. When inserted into the vagina using a specially designed applicator, LACTIN-V helps reestablish the population of…
New Anthrax Vaccine with Faster Protection
August 15, 2023The U.S. Food and Drug Administration (FDA) licensed CYFENDUS™, a novel anthrax vaccine developed with extensive support from NIAID and federal collaborators. Also known as AV7909 the two-dose vaccine offers faster protection than the previously approved three-dose anthrax vaccine, BioThrax. The licensure is an important step by the U.S. Government to prepare for intentional or naturally…
Point-of-Care Diagnostics for Common Sexually Transmitted Infections
April 10, 2025NIAID supported a clinical study of the Visby Medical Sexual Health Test, a palm-sized, rapid point-of-care diagnostic that can detect trichomoniasis, chlamydia, and gonorrhea infections in women using a self-collected vaginal swab. The highly sensitive, nucleic acid amplification-based PCR diagnostic returns results in less than 30 minutes, allowing patients to receive an accurate diagnosis…
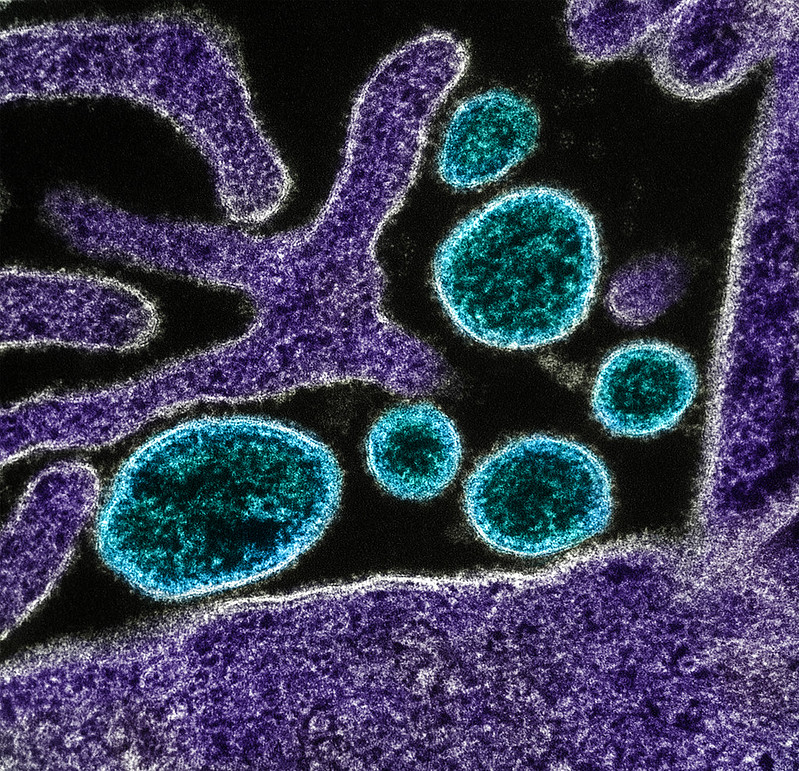
The First Approved Treatment for COVID-19
December 13, 2024After the emergence of SARS-CoV-2, NIAID efforts played a critical role in accelerating the development of new therapeutics and other countermeasures. In January 2020, the first cases of COVID-19 were reported in the United States. One month later, the Adaptive COVID-19 Treatment Trial (ACTT) began. Sponsored by NIAID, this was one of the first clinical trials for an experimental COVID-19…
An Antibody Test for COVID-19
December 13, 2024NIAID provided support for one of the first serologic tests for COVID-19, a two-step Enzyme-Linked ImmunoSorbent Assay (ELISA) which was developed by researchers at the Icahn School of Medicine at Mount Sinai.
A New Rapid Diagnostic for Melioidosis
December 23, 2024NIAID-funded researchers have developed a new rapid diagnostic for the tropical disease melioidosis, a disease caused by the bacterium Burkholderia pseudomallei. Melioidosis affects approximately 165,000 people worldwide, mainly in Southeast Asia and northern Australia, and causes 89,000 deaths annually.
Intravenous Fosfomycin to Treat Multidrug-Resistant Infections
May 13, 2022NIAID provided support to Zavante Therapeutics, Inc. (now Nabriva Therapeutics) for the clinical development of the intravenous (IV) form of the antibiotic fosfomycin (ZTI-01). Fosfomycin is a broad-spectrum antibiotic active against many Gram-positive and Gram-negative bacteria, including multi-drug resistant strains.
Treating Bed Nets to Prevent Malaria
November 15, 2024A NIAID-funded research team, led by scientists at Harvard University, exposed Anopheles gambiae mosquitoes, the main vectors of malaria in Africa, to low concentrations of atovaquone (a malaria drug). Treating bed nets with an antimalarial, such as atovaquone, could significantly counteract mosquito insecticide resistance as well as transmission of malaria to humans. Research is ongoing to…

Rapid Diagnostics to Combat Antimicrobial Resistance
November 15, 2024NIAID supports a number of diagnostics to combat antibiotic resistance, including multiplex platforms such as the FilmArray Blood Culture Identification Panel by BioFire Diagnostics LLC, an affiliate of Biomérieux.
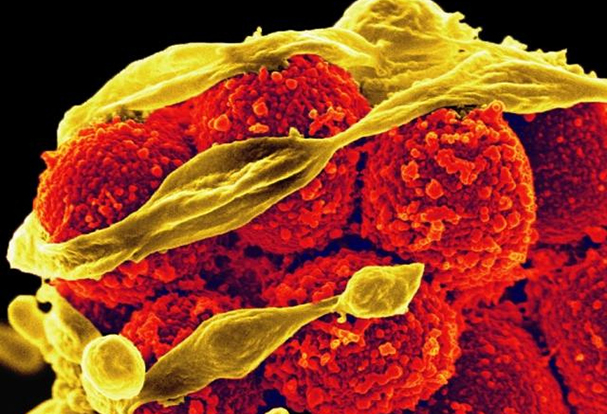
Designer Flu Proteins: A New Approach to Universal Influenza Vaccines
December 13, 2024NIAID is supporting research to promote the development of universal influenza vaccines, which would provide broader protection against multiple strains of influenza viruses.
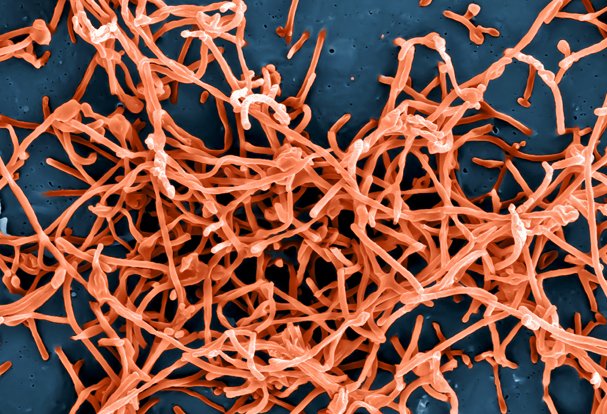
Rapid, Accurate Diagnostics for Ebola
November 21, 2024NIAID supported development of diagnostics to detect Ebola virus infection, including rapid identification and can be deployed at the point-of-care where Ebola outbreaks occur. Two diagnostics became available under Emergency Use Authorization (EUA) by the Food and Drug Administration for detection of the Ebola Zaire strain during the 2014 West Africa outbreak.
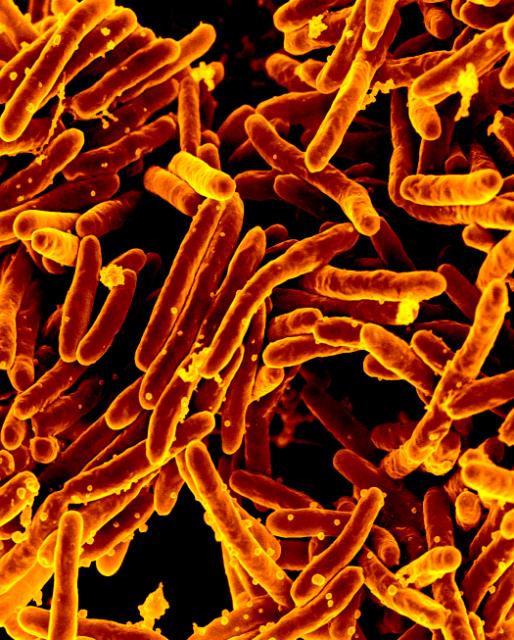
NIAID Enables Approval of Novel Anti-TB Drug
December 13, 2024The U.S. Food and Drug Administration (FDA) approved a new anti-tuberculosis (TB) drug, pretomanid (PA-824).
Synthetic Tetracyclines To Combat Bacterial Infections
May 13, 2022Tetracyclines are broad-spectrum antibiotics used to treat bacterial infections, but many bacteria are developing resistance to treatment. NIAID is supporting the development of novel tetracyclines that are not subject to existing tetracycline resistance mechanisms and therefore represent important new tools for the treatment of multidrug-resistant (MDR) bacterial infections.
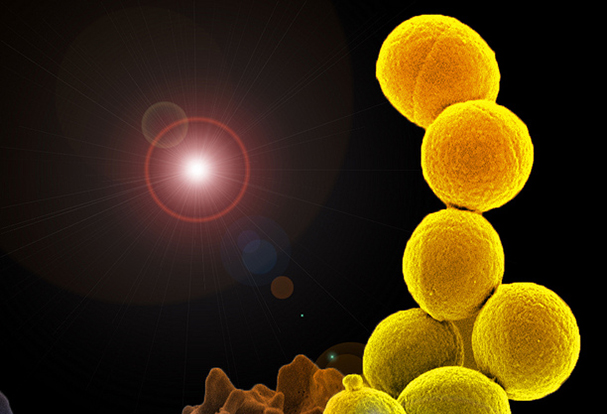
Vaccine Candidate Active Against Candida and Staphylococcus aureus: NDV-3
May 13, 2022NIAID is supporting a vaccine candidate that is being developed to protect against Candida or Staphylococcus aureus infections.
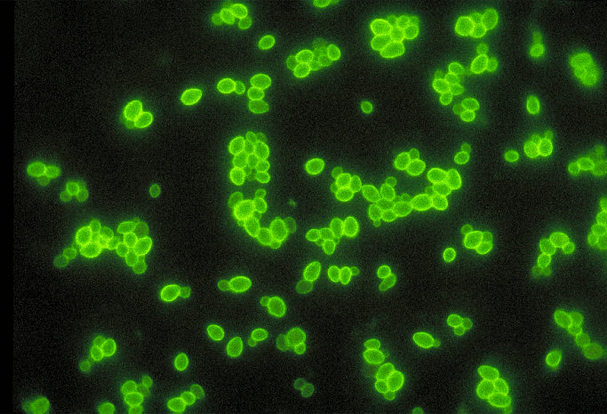
Second-Generation Smallpox Vaccine: Modified Vaccinia Ankara (MVA)
November 19, 2024NIAID recognized the need for a safer smallpox vaccine than Dryvax and ACAM2000 that could be used to protect patients with weakened immune systems, like those with HIV or cancer. A second-generation vaccine using Modified Vaccinia Ankara (MVA)-a highly weakened vaccinia virus that does not replicate well in humans is being developed by Bavarian Nordic.
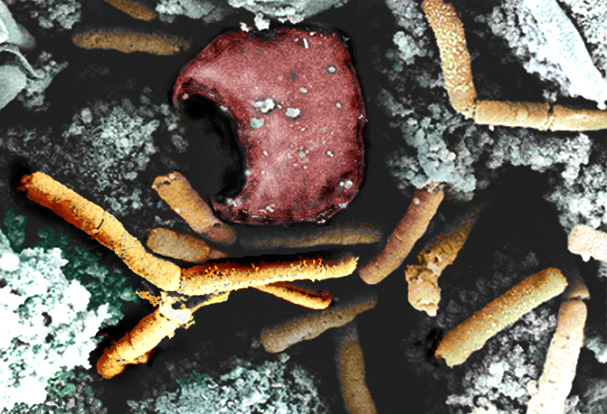
A New Treatment for Inhalation Anthrax: Anthim
November 19, 2024NIAID, in coordination with the HHS Biomedical Advanced Research and Development Authority (BARDA), supported preclinical and clinical research to develop several antibody-based therapeutics as anthrax antitoxins. One such product is Anthim, a human monoclonal antibody produced by Elusys Therapeutics, Inc.
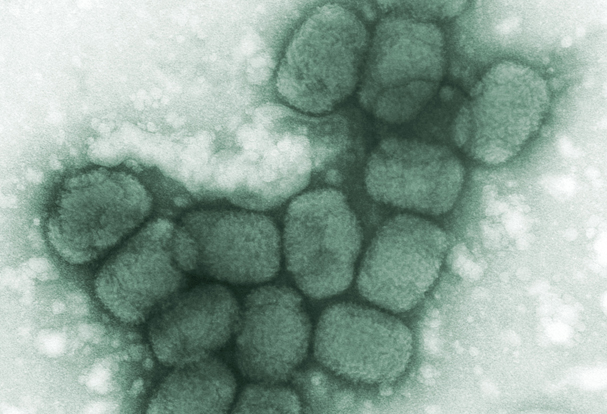
A Novel Vaccine and Therapeutic for Hendra and Nipah Viruses
May 13, 2022NIAID-funded researchers at the Uniformed Services University of the Health Sciences (USU) and their collaborators at the National Cancer Institute discovered a potential antibody treatment for Nipah and Hendra virus. The researchers developed a human monoclonal antibody (mAb) known as m102.4 that targets the G glycoprotein of both viruses and found that the mAb effectively protected ferrets…