Edition:
NIAID Funding News provides funding, policy, and other information to NIAID's extramural research community and Institute staff. Visit Stay Informed About Policy Changes and News for more opportunities to connect with us.
Changing Institutions? Steps to Take for a Grant to Go with You
If you are conducting human subjects research or research using vertebrate animals, you’ll need to begin the institutional review board or institutional animal care and use process, respectively, at the new institution.
When a Modular Budget Is Right for You
Request a budget sufficient to make your proposed project successful; reviewers will not respond well when faced with a budget that is clearly inflated or insufficient for the proposed work.
Don’t Derail Your Application: Avoid These Electronic Submission Errors
Submitting your application well in advance of its receipt date will allow you ample time to validate the upload and correct any content-related upload errors.
Tabulating NIAID’s R01 and R21 Application and Award Counts for FY 2024
Each year, we share the number of R01-equivalent and R21 applications that NIAID received in the previous fiscal year as well as the number of grants NIAID awarded.
Don’t Waste Your Time: Take an Extra Minute When Selecting NOFOs, Form Sets
We’ve now reached a stretch of time in which two form sets (i.e., FORMS-H and FORMS-I) and two versions of certain notices of funding opportunities (NOFOs) will be active simultaneously.
How to Use the World’s Largest Public Clinical Trials Registry as a Resource
While many researchers treat ClinicalTrials.gov only as a repository to submit required study information, it’s also worth thinking of it as an extensive online library.
Long COVID and the RECOVER-TLC Initiative
Long COVID’s broad and varied effects on diverse populations demand new clinical approaches and fewer restrictions to inclusion and access to achieve breakthrough results.
NIAID Sets Interim Paylines, Financial Management Plan for FY 2025
Remember, paylines are funding cutoff points for investigator-initiated grant applications based on score during peer review. Notably, we’ve already set interim paylines for every relevant award type.
Happenings from the September Meeting of NIAID’s Advisory Council
NIAID Director Dr. Jeanne Marrazzo shared remarks before NIAID's Advisory Council, as did Dr. Ted Piersen, Director of NIAID’s Vaccine Research Center.
What Is the NIAID Advisory Council and What Does It Do?
NIAID's Advisory Council is comprised of both experts in health and science, contributing technical expertise and an understanding of the needs of research communities of academia and industry, and lay members, who impart a perspective of people and communities affected by diseases in the NIAID research mission.
Explore NIAID Topics for Small Business Innovation Research Contract Solicitation
The annual NIH solicitation serves as a vehicle for offerors to propose research projects on a multitude of scientific topics from across NIH. Proposals are due October 18, 2024.
Small Business Research: Priority Funding Topics for 2025
NIH assembled a list of scientific priorities for small business awards that match the research interests of its institutes and centers, including NIAID.
Check Whether Your Research Is a Good Fit for ARPA-H
The Advanced Research Projects Agency for Health (ARPA-H) aims to advance high-potential, high-impact biomedical and health research that cannot be readily accomplished through traditional research or commercial activity.
Looking to the Future at the June Meeting of NIAID’s Advisory Council
NIAID Director Dr. Jeanne Marrazzo shared remarks before NIAID's Advisory Council, as did Dr. Steven Holland, Director of NIAID's Division of Intramural Research.
Explore Spending Estimates and Public Health Burden by Disease Category
NIAID often encourages researchers to use NIH RePORT tools—including Research, Condition, and Disease Categorization (RCDC)—to identify potential collaborators.
Bookmark NIH Webinars Page for Upcoming and Past Events on Funding Topics
For recently established policies, watching a webinar may prove an easier path to understanding NIH's new procedures than would reading policy announcements.
Learn About NIAID Career Opportunities and How to Apply
While many jobs at NIAID require expertise specific to our area of science, you'll notice that many position titles and functional roles are often similar across NIH’s many institutes and centers.
Explore the World of Research and Development Contract Solicitations
Contracts are government requirements for a product or service with specific goals, deliverables, and deadlines, and work done under a contract requires programmatic oversight by NIAID staff.
FORMS-I Transition: Setting Up Dominoes to Topple on January 25, 2025
NIH will transition to FORMS-I for application due dates on or after January 25, 2025, and also implement new policies and procedures that accompany the switch.
Some Applications Have Deadlines for Actions Before the Due Date
NIAID staff can identify issues that may cause your application to be withdrawn, not fare well in peer review, or have lowered potential for an award. The earlier you contact us, the earlier we can provide advice that you can use to improve your application.
Know How to Demonstrate Scientific Progress in Annual Reports
Your program officer will assess the progress, delays, and planned next steps you describe and compare that to your budget request and justification for approval.
Examining NIAID’s R01 and R21 Application and Award Counts for FY 2023
We share the number of R01-equivalent and R21 applications that NIAID received in the previous fiscal year as well as the number of grants NIAID awarded. To add context, we present the data alongside the same figures for the preceding 4 years.
New Leaders Take Center Stage at Advisory Council Meeting in January
This was the first meeting at which NIAID Director Dr. Jeanne Marrazzo shared remarks before NIAID's Advisory Council. It was also the first time that guest speaker Dr. Monica Bertagnolli, NIH Director, addressed our Council.
NIAID Uses Selective Pay, Bridge Awards to Complement Top-Scoring Applications
NIAID strives to always fund those applications that score best in peer review, but we also discern which applications outside the payline can help guard against scientific gaps emerging in our research portfolio.
Know Your Timeline to Award If All Goes According to Plan
Knowing when NIAID will begin funding your project is essential—it’s necessary for hiring support staff or arranging subcontracts or even timing a renewal application.
Don’t Be Fazed by Phased Awards
Submitted applications contain plans for two phases of research, including milestones that the applicant believes will justify continued support into the second phase.
NIH or NIAID? Know the Difference
NIH is made up of 27 institutes and centers, each with a specific research agenda, often focusing on particular diseases or body systems.
Promptly Determine Whether You’re Eligible to Apply
While a notice of special interest (NOSI) may stipulate eligibility criteria within its Purpose section, most NOSIs defer to the instructions listed in the identified notices of funding opportunities.
Why Criterion Scores Don’t Add Up
The criterion scores reflect the views of the assigned reviewers while your overall impact score reflects the scores of all the panel members who voted.
A Summary of Outcomes from Our September Advisory Council Meeting
NIAID Acting Director Dr. Hugh Auchincloss provided remarks on the Institute’s administrative, budget, and scientific news. He was followed by guest speaker Dr. Ted Pierson, Director of NIAID’s Vaccine Research Center, who discussed recent scientific advances and ongoing research priorities.
Explore NIAID Topics for Small Business Innovation Research Contract Solicitation
NIH’s Small Business Education and Entrepreneurial Development program will host an HHS SBIR Contracts Solicitation webinar to discuss the opportunity on September 27, 2023, at 1 p.m. Eastern Time.
Inform the Center for Scientific Review of Your Assignment Preferences
By using the optional PHS Assignment Request Form, you can convey your preferences for which NIH institutes or centers and study sections are ideal to receive your next application.
How NIAID Sets a Payline—A Conservative Approach in Light of Uncertainty
We can increase a payline and then fund applications from earlier in the fiscal year that are now within the payline, but we cannot decrease a payline and pull back funding from awards already made.
Explore Our Priority Topics for Small Business Research Projects
To accompany the annual small business parent notices of funding opportunities, NIH assembles a list of scientific priorities for small business awards of interest to its institutes and centers.
Grant Coming to an End? Avoid and Survive a Funding Gap
As long as you have accomplished enough to build a strong case for renewing your grant, apply early so you have time to revise and resubmit without risking an interruption in funding.
New Form to Disclose Foreign Relationships for Small Business Concerns
NIAID will require small business innovation research and small business technology transfer applicants to provide a “Required Disclosures of Foreign Affiliations or Relationships to Foreign Countries” form before issuing a grant award.
Highlights from June’s Advisory Council Meeting
NIAID Acting Director Dr. Hugh Auchincloss discussed administrative, budget, and legislative updates, as well as recent scientific findings. Then Dr. Steven Holland, Director of NIAID’s Division of Intramural Research, described key research accomplishments at our intramural laboratories.
Successful Sample Applications Demonstrate Good Grantsmanship
To observe how other applicants approach grantsmanship, examine the text sections of the successful example applications—particularly the Specific Aims and Research Plans.
Quiz Yourself on NIH Grant Funding Terminology
How familiar are you with NIAID’s application and grant terminology? Test your knowledge by taking our quiz and pay attention to the explanations for each answer.
There’s More to Biosafety and Biosecurity Than Select Agents
Review key terminology like dual use research of concern, enhanced potential pandemic pathogens, select agents, and recombinant or synthetic nucleic acid molecules.
Address Just-in-Time Requests and Bars to Award Quickly
While most of the just-in-time information we request is routine, be aware that certain codes and comments in your summary statement can indicate a funding restriction or bar to award.
Highlight Preliminary Data in Your Next Application
Focus on what reviewers would like to see in your application: enough information to convince them that your proposed project can be accomplished and is likely to have a high impact.
Generate Your Own Lists of Recently Funded Grants
You can create a list of recently funded NIAID grant awards to better assess the direction of preferences and priorities for NIAID peer reviewers and program staff.
Stay on Top of SAM Renewals and Organizational Registrations
NIAID cannot provide grant funds to organizations with an expired System for Award Management (SAM) registration; this includes competing and noncompeting awards.
Reviewing NIAID’s Application and Award Counts for FY 2022
We share the number of R01-equivalent and R21 applications that NIAID received in the previous fiscal year (FY) as well as how many awards NIAID made in turn.
Appreciation for 2022 NIAID Peer Review and Committee Volunteers
On behalf of NIAID and our research community, we express our deepest gratitude to fiscal year 2022 review and advisory group volunteers for donating their precious time and effort.
Top Ten Things NIH RePORT and RePORTER Can Do for You
Use custom crafted queries to learn what active research projects represent the cutting edge of your field, find potential collaborators for future projects, and much more.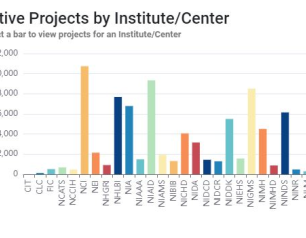
Remember to Register, Review, and Update Records at ClinicalTrials.gov
Those conducting an NIH-funded clinical trial must promptly update study records, including updates in the recruitment status or study completion date(s), made no later than within 30 days of the occurrence.
R21 Grants Make for Slippery Stepping Stones
The exploratory/developmental grant (R21) activity code provides up to two years of funding support. Typically, direct costs for the entire project period are capped at $275,000.
NIH and Tribal Nations—Commitment and Collaboration
Regular, meaningful engagement with Tribal partners helps NIH ensure American Indians and Alaska Natives are well-represented in research and benefit from its outcomes.
Extramural Research Overview for Fiscal Year 2020
NIH’s fiscal year 2020 appropriation totaled $41.6 billion. Of that total, NIH spent $30.8 billion to award 56,169 new and renewed extramural grants to 2,650 U.S. and international organizations.
Highlights From January 2021 NIAID Advisory Council Meeting
In his remarks to NIAID’s Advisory Council, NIAID Director Dr. Anthony Fauci shared key staff transitions, legislative updates, and COVID-19 news.
Writing a Winning Application—Write To Excite
Create a winning application by keeping your NIH peer reviewers in mind as you anticipate questions about your project's significance and innovation.
Writing a Winning Application—Consider the Review Committee
Match your research project to a review group with appropriate expertise and create an application that appeals to the reviewers and catches their interest by proposing concepts and preliminary data they would regard as innovative.
Taking a New Approach to Our Research Training Portfolio
NIAID is committed to improving the availability and effectiveness of our training, fellowship, and career development awards, to include increased support for certain research training activity codes.Adjustments to NIH Policy for Subawards and Implementation
This summer, NIH announced planned changes to its policy on subawards—arrangements in which a grant recipient has another organization perform grant-supported research activities as part of the federal award.
Opportunities and Resources
We aim to increase understanding of these mechanisms in order to identify candidate markers of disease progression as well as potential targets for immune-modulatory treatment to decrease TB and HBV risks in people living with HIV.
The cooperative research program will support centers that integrate clinical and translational research to conduct studies on mechanisms underlying the onset and progression of asthma and allergic diseases.
This notice of funding opportunity supports basic research on HIV-1 Env biology, and/or translational research that develops or optimizes biologics.
NIAID will support the development of safe and effective long-acting/sustained release technologies to help prevent and treat HIV and related co-infections such as tuberculosis, hepatitis B, and hepatitis C.
NIAID will support multidisciplinary, collaborative research programs focused on discovery to early development research to inform new approaches to prevent, diagnose, and treat antibiotic-resistant bacterial infections.
Propose a multidisciplinary research group that will implement phage therapeutic research by focusing on preclinical development and study pharmacokinetics and pharmacodynamics.
NIAID seeks to capitalize on the robust herpes simplex virus basic research field and to encourage more researchers to advance products into the development pipeline.
We seek milestone-driven, early-stage translational research focused on drug discovery and development of novel therapeutics against select fungal pathogens including Candida species that remain a clinical challenge.
Propose to discover and develop novel therapeutics directed at intracellular HIV targets and eliminate viral proteins by mechanisms like protein degradation, targeting of viral RNA through inhibiting RNA processing as well as degradation.
This opportunity is designed to support cohorts of the Pediatric HIV/AIDS Cohort Study—a streamlined and agile grant program that addresses developmental and clinical issues that affect individuals with HIV.
Projects should include meaningful engagement with implementing partners such as public health departments, healthcare organizations, and other service providers, as well as community members and people with lived experience.
Greater knowledge of the interactions and factors driving tuberculosis transmission would allow efficacious approaches for preventing transmission to be developed or improved and adapted for broad scale-up.
This opportunity encourages discovery and development of small molecule compounds with activity against viral targets that could be administered orally as monotherapy or in combination with other drugs to treat infections caused by viruses of pandemic potential.
Demonstrate innovative approaches to data reuse, explore strategies for leveraging data across data resources, and identify methods to ensure effective integration of data obtained through secondary analysis.
NIAID is interested in supporting research programs that focus on HIV and other health outcomes in women to inform and enable more targeted and effective HIV prevention and treatment.
Through a new initiative, we aim to fund hypothesis-driven research to investigate and improve the durability of immune responses to candidate vaccines for HIV prevention.
Apply to investigate the relationship between genetic variation in human leukocyte antigen and killer-cell immunoglobulin-like receptor region genomics and immune-mediated diseases.
NIAID will support studies that examine the impact of hormone therapy and antiretroviral drugs on treatment and prevention methods of HIV and other sexually transmitted infections.
Build a practical clinical research platform for rapidly and efficiently profiling participants’ intact, rebound-competent HIV reservoirs and their immunologic profiles.
NIAID will support basic research to elucidate mechanistic differences in susceptibility to chemicals of concern between pediatric and adult populations as well as applied research towards the discovery and early development of pediatric-safe medical countermeasures.
Each award’s principal investigator should be an established investigator capable of providing administrative and scientific leadership to the development and implementation of a career development program.
The notice of special interest (NOSI) encourages grant applications from Small Business Concerns to develop commercializable tools, resources, and approaches to capture the effects of climate change and the associated impacts of extreme weather events on human health.
Apply for research support to study basic mechanisms and biomarkers of trained immunity (i.e., innate immune memory), plus the functional implications of trained immunity.
The NIAID Clinical Trial Implementation Cooperative Agreement notice of funding opportunity (NOFO) is designed for high-risk trials. The NIAID SBIR Phase II Clinical Trial Implementation Cooperative Agreement NOFO allows small business concerns to proposed investigator-initiated clinical trials.
NIAID aims to better understand the mechanisms by which bats regulate immune responses to clear infection and prevent or resolve excessive inflammation.
3D organotypic culture models offer improved modeling of tissue architecture, cell-cell interactions, and other microenvironmental aspects of tissues and organ systems.
NIAID seeks research projects to discover and develop compounds that promote targeted protein degradation of viral, bacterial, parasitic, or fungal pathogen components, as well as non-protein targets.
Research the impact of host and viral heterogeneity on pathogenesis of disease, viral persistence, and immunopathology of Hepatitis B virus (HBV) and inform cure strategies for HBV in people living with HIV.
Apply if you can expand or improve engagement and re-engagement in HIV prevention, testing, treatment, and care services, or improve strategies to deliver integrated HIV prevention, treatment, and care services to address co-morbidities and co-infections.
NIAID will support basic research into novel mechanisms that contribute to intervention-mediated viral control and reductions in reservoir size as well as experimental, innovative targeted intervention activities.
Help address a deficiency in genetic tools and culture conditions that has blocked the development of medical countermeasures for select human eukaryotic pathogens.
Our new planning grant program will accelerate next-generation treatments for HIV and HIV-associated comorbidities, co-infections, and complications, and preventative strategies for HIV.
Understanding immune evasion by pathogens may be a critical factor in improving the design or utilization of prevention strategies, diagnostics, and treatments for tickborne diseases.
NIAID aims to support multidisciplinary Chemical Countermeasures Research Program-funded investigators through the development of infrastructure and exposure protocols for selected chemicals of concern.
Help us advance and improve congenital and adult acquired syphilis research. We encourage translational diagnostic development research including the collection and use of clinical samples.
NIAID will fund studies to develop vaccine candidates against enteric viruses that cause gastroenteritis in infants and young children, immune-compromised and -suppressed people, and elderly people.
Through this initiative, our aim is to facilitate the movement of exceptional postdoctoral fellows into independent positions within an accelerated timeframe, and further support them as talented, newly independent faculty.
Have you observed radiation-associated sex differences in your early-stage research to advance animal models, medical countermeasure development, safety, or biomarker science?
Apply if you are an international investigator in a resource-constrained country and can propose high-priority, regionally relevant infectious diseases research.
Apply if you can conduct research focused on less-studied emerging and re-emerging neurotropic viruses for which there are currently no approved vaccines or therapeutics.
Conduct epidemiological and observational research projects on the long-term cardiopulmonary sequelae following treatment for tuberculosis in persons living with and without HIV infection.
A set of new initiatives will create research centers in support of the Research and Development of Vaccines and Monoclonal Antibodies for Pandemic Preparedness (ReVAMPP) Network.
Through its new Medical Scientist Partnership Program, NIH will sponsor predoctoral students pursuing both a clinical doctoral degree and a biomedical research doctoral degree.
This initiative will focus on advanced vaccine development for STI pathogens that have limited candidates in the product development pipeline: Neisseria gonorrhoeae, Chlamydia trachomatis, and Treponema pallidum.
NIAID established the clinical trial network Consortium for Food Allergy Research to address concerns about the increasing incidence of food allergy and paucity of treatment options.
Successful offerors will help advance the research and development of promising candidate vaccines, therapeutics, and diagnostics for biodefense, emerging infectious diseases, and pandemic preparedness.
NIAID seeks to renew the Adjuvant Discovery Program, which supports the identification and characterization of novel, effective, and safe vaccine adjuvants.
Our aim is to facilitate the movement of exceptional postdoctoral fellows into independent positions within an accelerated timeframe, and further support them as talented, newly independent faculty.
The program provides comprehensive quality assessment evaluations for virologic assays for HIV and other viral pathogens performed on samples collected from participants enrolled in NIAID-sponsored and collaborative multisite clinical studies.
Propose research studies to explore the mechanisms of action of combination adjuvants that have already been shown individually to be effective in enhancing or modulating immune responses when compared with an antigen alone.
This notice of funding opportunity seeks research to characterize the role of defective HIV in blood and tissue sites in people living with HIV on antiretroviral therapy.
NIAID aims to foster new and innovative scientific endeavors related to universal influenza vaccine research that advance the major research areas defined in our Strategic Plan for the Development of a Universal Influenza Vaccine.
While most of our understanding of Post-TB Lung Disease (PTLD) comes from long-term human cohorts, using animal models for PTLD development could help address difficult mechanistic questions when relying solely on human studies.
Gather preliminary data around the role of understudied proteins associated with rare diseases and characterize new targets for treatment of human disease among them.
Find support for early- to mid-stage research and development of extracorporeal systems that mimic human responses and test appropriate medical countermeasures to treat civilian populations.
Submit an application to help foster, stimulate, and expand research on HIV/AIDS, HIV/AIDS comorbidities and co-infections, HIV/AIDS-associated implementation science, and HIV/AIDS-associated data science.
This initiative funds federally recognized American Indian/Alaska Native (AI/AN) tribes, tribal colleges or universities, tribal health programs, or tribal organizations to support health-related research, research career enhancement, and research infrastructure enhancement activities.
NIAID will help support and advance a human organ and tissue resource to provide a wide variety of human tissues and organs, diseased and normal, young and aged, to investigators for laboratory studies depending on research needs.
Projects will focus on novel ideas in radiation research, specifically medical countermeasures, biodosimetry, or animal model development to diagnose, mitigate, or treat injuries sustained during a radiation mass casualty incident.
Apply for funding to research immunologic events in vertebrate hosts that occur at the bite site (skin) and systemically during and after feeding by hematophagous and ectoparasitic arthropods.
Investigate and advance the mechanistic understanding on how immune ontogeny and functionality in early life could be harnessed in the context of HIV vaccines and broadly neutralizing antibodies to protect against acquisition of HIV infection.
Conduct fundamental research to characterize the acute or long-term pathophysiological effects of toxic chemical exposure on the pulmonary, ocular, or neurological system.
This initiative aims to strengthen the research capacity of institutions in low- and middle-income countries to investigate methods for preventing, treating, and controlling infectious diseases that pose significant health risks.
Apply if you can conduct research to capacitate, transform, and scale the delivery of HIV testing, prevention, and care services through pharmacists and pharmacies in U.S. or global settings.
This opportunity will support milestone-driven projects focused on developing novel predictive models, assays, tools, or platforms aimed at gaining a better understanding of the rules and compound properties that govern the penetration and efflux of drug-like small molecules into Gram-negative bacterial pathogens.
The Immunobiology of Xenotransplantation Cooperative Research Program supports research in preclinical nonhuman primate and human decedent models of porcine pancreatic islet, kidney, heart, lung, or liver xenotransplantation.
The Centers for Research in Emerging Infectious Diseases Network works to expand knowledge of re-emerging and emerging infectious diseases around the globe.
Through this notice of funding opportunity, you can receive funding to pay for national, international, and regional conferences, meetings, and workshops. Keep in mind, this award is not meant to fund an individual’s travel to a conference; rather, the funds are provided to the conference organizer.
A new Center will perform activities to coordinate the modeling community through coordinating cores and conduct research projects to advance modeling across and between scales of infectious and immune-mediated disease.
The solicitation’s purpose is to support manufacturing of material to advance preclinical, nonclinical, and Phase I/II clinical studies (e.g., vaccines, other biologics, vaccine components, and reagents).
A new opportunity supports radiation research at all stages of development for identifying biomarkers of injury and developing assays or devices for the purpose of triage, including assessing absorbed dose or predicting health outcomes of acute or delayed injuries.
Focus on ethical, legal, and social implications (ELSI) research relevant to NIAID’s scientific mission: HIV/AIDS or its comorbidities; infectious diseases; organ transplantation; and ELSI of clinical trials and implementation science.
Conduct an innovative project to study the mechanisms and management of vaccine or antibiotic drug allergy, including research on allergic responses to anti-viral, anti-fungal, and anti-parasitic drugs.
Through courses for skills development and mentoring activities, NIAID strives to support current and future research investigators from diverse backgrounds.
This initiative will stimulate the optimization and evaluation of long-acting drug delivery systems formulations in suitable preclinical animal models at an earlier age of adult drug development.
Request support for multi-component, multidisciplinary projects that address scientific questions relevant to AIDS prophylactic vaccine discovery research.
Help discover innovative methods and strategies to support the eradication of Rheumatic Heart Disease, which remains endemic in low- and middle-income countries and low-resource settings.
Successful applicants will demonstrate that they have the appropriate expertise, resources, and infrastructure needed to conduct advanced diagnostic evaluations at their site.
Fellowship grants provide stipends, tuition and fees, and an institutional allowance. Funding levels will vary depending on the candidate’s experience and proposed Research Plan.
Use study samples to gain a deeper understanding of mechanisms contributing to the development of allergic diseases, disease endotypes, and molecular targets for disease prevention.
The notice of funding opportunity helps Phase II and Phase IIB projects' progression to the commercialization stage by providing additional support for technical assistance and later stage research and development.
To apply, you must propose a research project integral to the approved, ongoing research of the parent award, with the potential to contribute significantly to the career development of the candidate.
Propose basic, preclinical, or clinical research studies using existing human samples to analyze and compare HIV reservoir establishment, dynamics, persistence, and post-treatment control among different HIV subtypes and in diverse cohorts of people living with HIV.
The clinical trial planning grant (R34) supports planning, design, and preparation of the documentation necessary to implement an investigator-initiated clinical trial.
Improve our understanding of the roles and interactions of immune cells at the maternal-fetal interface that support pregnancy and enable optimal placental development and function.
Conduct mechanistic studies of early-stage Mtb infection in the airway and lungs with and without HIV to identify interventional targets for vaccine and host-directed therapies.
The initiative supports early discovery of novel exploratory vaccine strategies to prevent HIV infection, as well as worthy basic vaccine discovery research that has not yet been fully exploited.
Collaborative research centers will help provide the foundation and infrastructure to complete basic, translational, and mechanistic clinical trials focused on Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS).
In your application, describe plans to develop either small animal models or surrogate virus-host systems for hepatitis B virus or hepatitis C virus research.
NIAID will support research to improve safe and effective precision therapeutics for pregnant and lactating persons, fetuses, neonates, and children, including those with disabilities.
The Centers for AIDS Research program fosters synergy and research coordination, supports emerging research opportunities, and promotes resource sharing among institutions that receive significant HIV/AIDS funding from NIH.
NIAID is soliciting contract proposals from offerors capable of providing a broad range of services to investigate vaccine formulations with HIV immunogens and adjuvants.
The health conditions of interest to NIAID impact the health of sexual minority and majority populations, and the Institute endorses meaningful sexual orientation and gender identity measurement in research, clinical care, and administrative settings.
The Physician-Scientist Pathway to Independence Award is designed to increase and maintain the field of new and talented physician-scientists by supporting mentored research training and an independent research period.
NIAID seeks a partner to operate the Cellular Immunology Core Laboratory, which will conduct, analyze, develop, optimize, and validate cellular immunologic assays for multiple pathogens, to be performed on fresh and frozen preclinical samples.
Conduct research on human immune system regulation and function to discover and characterize new principles of human immunology for preventing and treating infectious diseases, immune-mediated pathogenesis, and immune-mediated diseases.
The Mycobacterium tuberculosis (MTB) Quality Assessment Program will serve NIAID-sponsored and collaborating clinical trial networks and cohorts as well as individual grantees conducting research in and outside the United States.
Three initiatives cover distinct topics: pathobiological mechanisms of post-acute sequelae, pediatric COVID-19 and respiratory viral co-infection, and vaccine hesitancy among health disparities populations.
NIAID aims to develop a range of approaches for studying bunyaviruses using both established and novel technologies, prioritizing vector competence, virology and pathogenesis, and human innate and adaptive responses to infection.
The cohort study platform will collect and make publicly available high-quality risk factor, viral suppression, and other outcome data on HIV and associated comorbidities in middle-aged and older adults in the United States.
The initiative promotes discovery of novel, improved candidates for treating Botulinum Neurotoxin (BoNT) toxicity, as well as platforms for the intraneuronal delivery of antitoxins to reverse BoNT intoxication.
Advance our understanding of the impact of a host’s microbial experience on the development and function of host immunity and provide the data necessary to encourage broader use of the mouse models in immunologic and microbiologic research.
Propose research on the fundamental mechanisms of toxicity of highly toxic Chemicals of Concern but also on potential new targets to inform the development of medical countermeasures that would be effective during and after civilian mass exposure situations.
Research civilian chemical medical countermeasures and novel treatment strategies to combat serious morbidity and mortality resulting from high consequence public health chemical emergencies.
Active grant awardees can receive support for in-scope research to reduce health inequalities among women who are understudied, underrepresented, or underreported in biomedical research.
Recruit, mentor, and support students, postdoctoral scholars, and eligible investigators from diverse backgrounds, including those from groups that have been shown to be underrepresented in health-related research.
Apply to organize a series of annual in-person scientific meetings, conferences, symposia, and workshops to advance innovations in therapeutic and chemical toxicology research.
Request support for basic and exploratory research projects that leverage recent scientific development to advance our understanding of Treponema pallidum bacterial pathogenesis.
Develop and validate use of Collaborative Cross mouse models to reproduce the impact of host genetic variations on human immune system responses, and screen and evaluate mouse lines for use in specific studies and disease models.
Apply for funding to conduct innovative basic, translational, and clinical research to identify and address epigenetic treatment strategies for achieving hepatitis B virus (HBV) cure in people living with HIV.
The Lasker Scholars program supports candidates in two phases: an initial phase within the NIH Intramural Research Program and a subsequent phase with extramural grant funding.
NIAID will establish a Coordination, Consultation, and Data Management Center and multiple Regional Consultation Hubs to support NIH's Ending the HIV Epidemic in the U.S. Initiative research portfolio.
Conduct product-focused research to advance controlled-release vaccine strategies shown to improve immune responses for HIV prevention, treatment, and cure, and also to develop simplified or single-shot vaccination formulations.
Research the causes and mechanisms of inborn errors of immunity, enable early detection and molecular diagnosis, and support strategies to treat and cure these disorders.
Apply to test and evaluate non-viral technologies capable of delivering RNA-based therapeutics into disease-relevant cells and tissues in vivo through a new notice of special interest.
Our Radiation and Nuclear Countermeasures Program seeks a contractor who can provide services and capabilities to maintain and expand a centralized dosimetry harmonization effort.
NIAID seeks applications from single institutions or consortia of institutions ready to generate, validate, and advance medical countermeasures against a select list of bacteria or fungi with known or emerging resistance to current therapies.
NIAID invites applications from actively-funded grant recipients to travel to NIAID laboratories for a short duration to perform research in partnership with a host intramural research investigator.
NIAID will fund research on late-stage engineering and preclinical development of innovative biological products that safely and specifically engage the immune system to kill HIV-infected cells.
Apply to study early islet dysfunction, autoimmunity initiation, and other metabolic perturbations present during the early stages of type 1 diabetes disease for which there are still few clinical interventions.
Our goal is to improve oversight of NIAID grant awards and compliance with NIH funding policies and federal research funding requirements for NIAID-supported foreign institutions.
Design interventions with consideration for sustainability within the communities where they are tested, and with the flexibility to be readily adapted, disseminated, and scaled up to other communities where culturally appropriate.
The Smart Health program supports high-risk, high-reward advances in computer and information science, engineering, mathematics, statistics, behavioral, and cognitive research to address pressing questions in the biomedical and public health communities.
Develop new medications by identifying novel research targets and lead molecules to develop therapeutic approaches that will be effective at various stages along the trajectory of opioid use disorders.
STRONG program funds can help you analyze your institution’s research capacity needs and strengths, then create an action plan to meet those needs and leverage areas of strength.
Aim to build on recent breakthroughs in biology of mucosal systems, biology and immunology of mucosal infection, and microbiome research.
Apply for funding to develop data science software that improves acquisition, management, analysis, visualization, and dissemination for data science research on infectious and immune-mediated diseases.
This program supports high throughput discovery and validation of novel B cell epitopes associated with infectious pathogens or vaccines against them, autoimmune diseases, allergens, or human leukocyte antigen epitopes associated with cell, organ, or tissue transplant rejection or tolerance.
Each center will create and provide access to interactive knowlegebases, innovative tools and software, and leading-edge expertise in bioinformatics.
Propose research to apply newly developed immunological tools and expand understanding of the immune response to vaccination at the molecular level.
Demonstrate that your clinical site has a track record of diagnosing rare and difficult-to-diagnose disorders, with appropriate expertise as well as the infrastructure and resources needed to conduct clinical evaluation and DNA sequencing of participants enrolled at your site.
NIAID aims to spur new approaches that target HIV transcription to shut down the expression of HIV RNA, proteins, and virus that occurs despite interruption of the viral life cycle by antiretroviral therapy.
NIH published three notices of special interest that each offer administrative supplements for ongoing research projects to further explore priority topics: climate health, software tools, and data readiness.
Can your organization develop, refine, and use animal and animal replacement models to assess candidate therapeutics, vaccines, and diagnostics targeted at infectious diseases and generate difficult-to-source reagents requiring an in vivo model?
The HIV Vaccine Trials Network (HVTN) invites outside investigators to apply to use Imbokodo/HVTN 705 participant specimens or study data to conduct independent scientific research. Respond by April 4, 2023.
By facilitating access to experienced mentors, relevant facilities, and protected time for training centered on nonhuman primate models, these awards will help early-career scientists address key issues in clinically relevant research areas.
The funds may be used to pay for computational services, supplies, equipment, and supported effort of additional scientific staff to sustain the research project while an investigator gives priority to a critical life event.
The Notice of Special Interest encourages rigorous interdisciplinary research on the complex intersection of women’s health, sex and gender, and social determinants of health.
NIAID's Division of Microbiology and Infectious Diseases will use this Broad Agency Announcement to advance the research and development of promising candidate therapeutics, vaccines, and diagnostics for biodefense and emerging infectious diseases.
Help ensure future effective global malaria control and elimination by focusing on “combination” vaccine concepts to improve efficacy, targeting one or more parasite antigens of the different life cycle stages of the parasites.
Predoctoral trainees should become capable of applying data science methods and technologies across infectious and immune-mediated diseases research domains.
Each consortium will advance and improve diagnosis, management, and treatment of numerous, diverse rare diseases through highly collaborative, multi-site, patient-centric, translational, and clinical research.
Apply if you can further the development of new products to prevent herpes simplex virus infection and improve the diagnosis and treatment of patients living with herpes.
NIAID will support integrated multi-project research programs applying emerging and improved technologies to develop innovative gene- or cell-based HIV cure approaches.
Propose a partnership of investigators and existing clinical study sites in tuberculosis (TB) endemic countries, to evaluate early-stage TB diagnostic tests, assays, and strategies.
NIH seeks research projects studying the ethical, legal, and social implications of human genetic or genomic research, including empirical qualitative and quantitative methods.
Apply to conduct mid-stage research developing candidate medical countermeasures (MCMs) already demonstrating efficacy to mitigate or treat radiation injuries.
Propose a computational model that is data-driven, macromolecular, or hypothesis-based and mechanistically-driven and generalizable to multiple types of immune perturbations.
Receive funds to establish formal partnerships with other research groups to achieve outcomes that would likely be impossible without the expertise, models, and facilities available at partnering institutions.
Plan to target mechanisms that are integral for the replication, growth, survival, or virulence of a pathogen, including toxins or toxin-related targets as novel anti-infectives.
NIAID has extended the application deadline for the broad agency announcement (BAA) Rational Systematic Characterization and Selection of Adjuvants for HIV Vaccine Candidates; proposals are now due on July 7, 2023, at 4 p.m. Eastern Time.
Help us develop and advance approaches in genomic technologies, computational tools, and large-scale data analysis of human pathogens and their interactions with the host and microbiome.
Applications are due for three distinct initiatives providing administrative supplements for research at the nexus of women’s health, sex and gender, and health disparity populations.
Study the epidemiology of viral HIV suppression and determine study approaches or evidence-based digital treatment interventions to reduce HIV transmission in Mexico and South and Central America.
Apply to conduct research on disease and health conditions that predominantly affect women, present and progress differently in women, or are female-specific.
In The News
If you are applying to a parent Fellowship notice of funding opportunity for the April 8 or May 7, 2025 Standard Due Dates, you must use the FORMS-H application forms and instructions.
NIAID will remain funded at the level of fiscal year 2024 appropriations through March 14, 2025, when the current continuing resolution will expire.
The new NIH policy mandates the submission of author-accepted manuscripts to NIH's PubMed Central without a 12-month embargo period.
NIH’s Advisory Committee to the Director (ACD) provides recommendations on program development, resource allocation, NIH administrative regulation, and other aspects of NIH policy.
The newly structured candidate section will ask for four personal statements: 1) a statement of professional and fellowship goals, 2) a statement of fellowship qualifications, 3) a self-assessment, and 4) a statement of scientific perspective.
A new initiative will support efforts to independently replicate significant lines of research and validate novel technologies across different scientific research areas in preclinical, translational, and technology development studies.
Recipients of grants and cooperative agreements are now required to include in their Research Performance Progress Reports responses to several new questions that align with the Final NIH Policy for Data Management and Sharing.
Last week, NIH completed a major overhaul of its Grants & Funding website. You can watch a video tour for a summary of key changes to the site’s organization and resources.
As of October 1, 2024, NIH’s eRA system will no longer automatically generate and send just-in-time email notifications to applicants whose overall impact scores are below a designated level.
NIH seeks community feedback on several recommendations made by the Advisory Committee to the Director Working Group on Re-Envisioning NIH-Supported Postdoctoral Training.
To be eligible, you must be a doctoral-level clinician or researcher who’s committed to conducting NIH-qualified clinical research. If NIH approves your application, we will repay up to $50,000 each year toward your qualified educational debt.
The draft Public Access Policy builds upon NIH’s history of providing public access to scholarly publications resulting from the research it supports and proposes additional steps to accelerate access.
NIH’s Advisory Committee to the Director (ACD) provides recommendations on program development, resource allocation, NIH administrative regulation, and other aspects of NIH policy.
When you complete your next Research Performance Progress Report, you may encounter new questions that address the NIH Data Management and Sharing (DMS) Policy.
Answer a request for information on topics that NIH should consider when developing the next NIH-wide Strategic Plan for Sexual and Gender Minority Health Research.
NIH announced Ruth L. Kirschstein National Research Service Award (NRSA) stipend levels for awards made on or after October 1, 2023, which saw their largest year-over-year increase since fiscal year 2017.
NIAID now has an appropriation for fiscal year 2024, a key development as we determine how many awards the Institute can support in this fiscal year.
The volume of awards that NIH is already obligated to fund each year can amplify, limit, or delay changes in success rates following a change in the overall budget.
To be accepted within an application, your video must demonstrate devices or experimental data with a temporal element, i.e., a need to show how something functions or occurs over time, or demonstrate movement or change.
Join us in our annual appreciation of NIAID peer review and committee members, then learn how to volunteer for a peer review group yourself.
NIAID is now updating its Institute-wide Strategic Plan, and we invite feedback from the extramural research community on the research priorities and crosscutting themes that will drive our Strategic Plan.
Our online database serves as a central resource for country-specific clinical research requirements for regulatory and ethics approval, clinical trial lifecycle, sponsor responsibilities, informed consent, investigational products, and specimens.
NIH’s Advisory Committee to the Director (ACD) members provide recommendations on program development, resource allocation, NIH administrative regulation, and other aspects of NIH policy.
Establishing an NIH-wide strategic plan will help the new Office of Autoimmune Disease Research create opportunities for synergistic innovation and implement cross-cutting research.
Peer reviewers are prohibited from using and sharing materials from a confidential peer review meeting, and doing so constitutes a serious breach of confidentiality.
NIH SEED has useful foreign risk case studies to demonstrate instances in which foreign involvement does or does not generate security risks.
Learn about those exceptional researchers whose projects are already funded through the NIAID New Innovators Awards program.
NIAID has not reduced competing or noncompeting awards in some time, but we do so now in light of budget uncertainty.
In a Plan for Enhancing Diverse Perspectives, you'll broadly consider how diverse perspectives can advance research outcomes, in addition to bringing equity and inclusion to the science they inform.
Science communication is rapidly evolving, and the growing use of preprints and the sheer number of published studies make it increasingly difficult to determine which findings are worthy of attention.
The single “Data Management and Sharing Costs” line item will no longer be required; NIH recognizes that DMS costs may occur in multiple cost categories.
NIH has expectations, policies, and requirements for recipient institutions to foster an environment free from harassment, including sexual harassment, discrimination, bullying, retaliation, and other forms of inappropriate conduct.
Share input on a proposal to encourage use of designated member review (DMR), DMR subsequent to full committee review, veterinary verification and consultation, and administrative handling of increase in previously approved animal numbers.
The virtual tour lets visitors from around the world learn about NIH’s mission, research, labs, and facilities by showcasing 20 in-depth tour stops on NIH’s campus.
Our revised Strategic Plan is structured around five strategic priorities that capitalize on recent advances in the field; each critical to the development and evaluation of knowledge and tools needed to end tuberculosis globally.
The changes address the consortium relationship that a grant recipient needs to establish with an organization receiving a subaward under the grant to ensure compliance with NIH requirements.
NIH received more than 800 responses to a request for information on simplifying peer review criteria, a vast majority of which supported most of the proposed changes.
NIAID sets a separate R01 payline for new and early-stage principal investigators (PIs) to ensure success rate parity between established and new and early-stage PIs.
NIH aims to recognize the transformative cultures, systems, projects, and processes developed by academic institutions to promote inclusive excellence and create environments that foster and value a culture of diversity, equity, inclusion, and accessibility (DEIA).
What are the gaps and barriers to developing, diversifying, and sustaining a diverse extramural scientific workforce and building a scientific pipeline to support the NIAID mission?
Use the Budget Data Comparisons table to compare the enacted funding levels for fiscal years 2022 and 2023 across multiple NIH institutes and centers, including NIAID.
Heed NIH's call to improve complete reporting of results of vertebrate animal and cephalopod experiments by using the Animal Research: Reporting of In Vivo Experiments checklist.
Congress restricts the amount of direct salary paid to an individual under an HHS award. Effective January 1, 2023, the salary limitation is set at a rate of $212,100 for 100 percent effort.
NIAID Acting Director Dr. Hugh Auchincloss provided updates on HIV/AIDS, malaria, Ebola, a promising Marburg vaccine, universal flu vaccine efforts, non-viral asthma attacks, monoclonal antibodies to target Epstein-Barr virus, and human antibody response to vaccinations.
NIH’s Advisory Committee to the Director (ACD) provides recommendations on program development, resource allocation, NIH administrative regulation, and other aspects of NIH policy.
The Revised Simplified Review Framework would reorganize five major regulatory criteria under three scored categories and reduce the number of non-score driving review considerations that reviewers evaluate.
We are starting fiscal year 2023 under a continuing resolution, which will run through December 16, 2022. Our budget officials are now calculating appropriate interim payline levels.
Paylines—either percentiles (R01s only) or overall impact score order—are the funding cutoff points for investigator-initiated applications.
The extramural community collectively wrote and submitted a higher volume of research project grant applications amid pandemic shutdowns in summer 2020.
NIAID Director Dr. Anthony Fauci discussed topics like budget and legislation, and Dr. John Mascola, director of the Vaccine Research Center (VRC), described VRC's work on mRNA and therapeutic antibodies related to COVID-19 as well as on influenza vaccines.
Submit a proposal centered on questions directly related to the Asia-Pacific region with potential to add to global knowledge about infectious diseases and immunology.
Unsure How to Link Your ORCID iD and eRA Commons Account? Watch This Video; New NIH Policy Ensures Patient Access to Certain Licensed Products; NIH Implements New Funding Opportunity Goals Text
NIH Releases Midcourse Review of the NIH-Wide Strategic Plan; New Resources Webpage for Researchers with Disabilities; Bookmark a Pair of NIH Informational Pages
NIH invites feedback that will help shape the framework of the ensuing NIH Strategic Plan for Disability Health Research for Fiscal Years 2026 to 2030.
Increased Interim Paylines for Fellowship, Career Development Awards; NIH Develops Training Resources for Responsible Conduct of Research; Enhance Your Presentations with High-Quality Science and Biomedical Art Illustrations
Investigators will soon be required to use Science Experts Network Curriculum Vitae (SciENcv) to complete Common Forms and the NIH Biographical Sketch Supplement to produce digitally certified PDFs for their application submission.
Note an Addendum to NIAID’s Financial Management Plan for FY 2025; HHS ASPR Posts FAQs on DURC/PEPP Policy; New Scientific Integrity Policies Focus on Public Trust
Last Call to Answer the NIAID Funding News Survey; Register Now for Small Business Entrepreneurship Boot Camp; Double Dipping Could Lead to Payback; Instruction for Labs Working with Synthetic Nucleic Acids
To aid researchers planning such studies around the globe, NIAID maintains the ClinRegs database of country-specific, clinical research regulatory information for 23 countries.
Join NIH policy experts for a webinar covering essential updates and ways in which the latest changes in NIH policies, systems, and resources might impact your institution’s grants administration procedures.
Foreign organizations that expend $750,000 or more in federal awards during their fiscal year must conduct either a single audit, performed in accordance with the requirements outlined in 2 CFR 200 Subpart F, or a program-specific audit.
Beginning on May 25, 2025, NIH will require grant applicants and recipients to use Common Forms for Biographical Sketch and Current and Pending (Other) Support, rather than the NIH-specific format pages currently in use.
NIH and NIAID prioritize support for early-stage investigators as a strategy to sustainably strengthen a robust and diverse workforce.
A central goal of NIH policies is to ensure that the demographics of participants in our clinical research will inform clinical practice to benefit people who are affected by the disease or condition under study.
Through a new request for information (RFI), NIH seeks input about engaging the public throughout all stages of clinical research; not solely during recruitment or participation in clinical research and trials.
Provide feedback on a draft policy and accompanying draft license agreement language that incorporates patient access in the commercialization process for NIH-owned inventions.
FDA and NIH are requesting comments regarding the clinical research terms related to innovative clinical study designs, including studies using real-world data to generate real-world evidence.
We announced a medley of changes for our career development and institutional training awards meant to help us better foster future investigators and maintain a balanced research training portfolio.
NIAID shares sample applications to provide examples of good grantsmanship and successful approaches to presenting a Research Strategy and Specific Aims.
Parent R01, R03, R21 NOFOs Will Now Expire in January 2025; Consider a New Perspective on Writing with Narrative Structure for Grant Applications; Remember May 27 Deadline for Input on NIAID’s Next Strategic Plan
The Cumulative Investigator Rate is an NIH-wide person-based metric, calculated as the number of unique principal investigators (PIs) who were designated on a research project grant (RPG) award, divided by the number of unique PIs who were designated on an RPG application over a 5-year period.
NIAID’s TB Portals Program is a global collaboration of clinicians, researchers, and data scientists united in the mission to eradicate tuberculosis.
Each National Research Service Award fellow or trainee is eligible to receive $2,500 per budget period toward expenses for childcare provided by a licensed childcare provider.
If a recipient does not submit all required closeout reports within a year of the period of performance end date, NIH must unilaterally close the grant and report the recipient's failure to comply with the terms and conditions of award.
NIH's Data Management and Sharing (DMS) Policy requires sharing of all scientific data, defined as all data necessary to replicate research findings regardless of whether the data are used to support scientific papers.
U.S. institutions can submit investigator-initiated grant applications and, if selected for funding, the Research Council of Finland may fully fund the Finnish investigators.
Subawards Policy Changes Take Effect on January 1, 2024; NIH Is Currently Operating Under a Continuing Resolution; Podcast Episodes Discuss the Hidden Curriculum for Early-Career Researchers
Dr. Monica Bertagnolli Is the New NIH Director; Attend Webinar for DEIA Excellence Supplemental Awards; Prepare Now for Upcoming Opportunity on Systems Modeling; Register for Native American Health Research Webinar on January 25; Monthly Automated Reminders for Rejected Final FFRs
A trans-NIH committee will develop a timeline and implement changes to improve review of Ruth L. Kirschstein National Research Service Award (NRSA) individual fellowship applications.
The draft NIH Scientific Integrity Policy establishes the appointments of, and roles and responsibilities for, the positions of NIH Chief Scientist and Scientific Integrity Official.
Join NIH Webinar on Subaward Rules and Procedures; Eligibility Window for F30 Applicants Is 48 Months from Matriculation; Attend NIAID Workshop on Approaches to Bacteria Antigen Discovery; Answer a Pair of RFIs from NIH’s Office of Data Science Strategy; Change to Organization Eligibility for STRONG Funding Opportunity
You can use our new tool to search across millions of public datasets to find infectious and immune-mediated disease data for reuse and reanalysis.
The Maximizing Opportunities for Scientific and Academic Independent Careers program supports researchers from diverse backgrounds through individual postdoctoral career transition awards and institutionally-focused research education cooperative agreements.
For help while you draft your application, consider recruiting colleagues from your institution and other scientific peers to volunteer to provide feedback on your application.
Survey results show that over 90 percent of reviewers reported the training modules were effective and they felt better prepared to take action afterwards.
NIH’s Continuous Submission Policy allows appointed members of review and advisory groups to submit applications to certain notices of funding opportunities after the standard deadline.
NIH’s Advisory Committee to the Director (ACD) held its most recent meeting on June 8 and 9, 2023. ACD members provide recommendations on program development, resource allocation, NIH administrative regulation, and other aspects of NIH policy.
NIH requests ideas for biomedical and behavioral research challenges and opportunities that may be of interest to the Common Fund for future investment.
NIH tasks peer reviewers with identifying the most promising trainees as well as excellent, individualized training programs that will help them become outstanding scientists.
NIH will employ and support up to five exceptional early-stage investigators who have not yet achieved tenure at a research institution.
The National Library of Medicine based ClinicalTrials.gov modernization efforts on user feedback to improve how visitors search, view, and download information about clinical trials.
NIH will allow applicants to submit preliminary data as post-submission materials for new applications (Type 1) if the notice of funding opportunity uses the R01, R03, or R21 activity codes and allows preliminary data.
You may be able to anticipate which standing review group and primary reviewers are most likely to review your application, then write to fit your reviewers' areas of scientific focus and levels of understanding.
Share your thoughts on the roles and responsibilities of academic postdocs, including challenges in recruitment, retention, and quality of life of postdoctoral trainees.
NIAID invites information and recommendations to enhance DEIA across the Institute’s funded activities, such as strategies to address any systematic and structural barriers to receiving NIAID research funding that may exist.
NIAID uses paylines as funding cutoff points for certain types of investigator-initiated grant applications. Last week, NIAID updated the interim paylines for research projects (R01), small grants (R03), and exploratory/developmental grants (R21).
The NIH Grants Policy Statement provides up-to-date policy instruction that serves as the standard terms and conditions of award for NIH grants, as well as guidance for applicants pursuing an NIH grant.
Use New Versions of Parent Announcements; Comment on Use of Metadata to Increase Transparency of Research Results; Updated eRA Resource Aligns Training Tables with New FORMS-I Requirements; Stream NIAID's Advisory Council Meeting on January 27.
HHS Policy Also Caps Indirect Salaries Paid Using Award Funds; Small Business Recipients—Get Help to Hone Your Product Pitch; Read Summary of Recent Artificial Intelligence Use Cases at NIH
With Gratitude to Dr. Hugh Auchincloss, and a Welcome to Dr. Sarah Read; Newcomer to NIH Funding? Attend the NIH Grants Process Primer Webinar; OLAW Annual Reports Are Due on December 1; Loan Repayment Program Application Deadline Is November 21
Reminder of NIH Policy on Late Applications Caused by Weather Emergencies; Examine Data on Professional Degree Types in NIH’s Grant Portfolio; NIH Revises Its Definition of Sexual and Gender Minority Populations
A Reminder for Authorized Organization Representatives to Verify Grant Assignments; NIAID Continues Participation in Ecology and Evolution of Infectious Diseases Program; Refresh Your Knowledge of NIAID and NIH Data Sharing Policies; Award Challenge Offers Prizes for Data Reuse Projects
Attend NIH Webinars on Grant Closeout, Fellowship Application and Review; Stay Sharp on Best Practices for Genomic Data Sharing; A Reminder for Small Businesses Seeking Funding in Women’s Health Research; Subscribe to New Quarterly Newsletter from COSWD for Funding Insights
Now Available: New Career Development Sample Applications; Hurry with Submissions for NIH Entrepreneurship Bootcamp; Distinguish the Priority Areas of NIH’s Institutes, Centers, and Offices
Change in Prior Approval Requirements for SBIR/STTR Recipients to Add a Subaward; Funding for Meetings to Support Data Harmonization of Autoimmune Disease Research
NIAID Strives to End the Use of Stigmatizing Language; Contract Vendors Can Submit Inclusion Data Directly into HSS; Nominate a Researcher for 2024 SGM Research Awards Program
Temporary Exemption of H5 Avian Influenza Viruses from Select Agents Regulations; NIH Sets Expectations for Upholding the NIH-Lacks Family Agreement; Answer Request for Information on All of Us Research Program Data
New Government-Wide Policy for Dual Use Research of Concern and Pathogens with Enhanced Pandemic Potential in May 2025; NIAID Sets Final R01, R03, and R21 Paylines for FY 2024; NIH Increases Childcare Support for NRSA Fellows and Trainees
Share feedback on existing immunology resources, future needs of the research community, and potential future directions of data and knowledge management.
Senior investigators should concern themselves with the career trajectory of promising HIV researchers, and at NIAID we are exploring ways to maximize our resources to fund as many worthy candidates as possible.
Register for April 17 Webinar on Simplified Review Criteria; NIAID Receives an Appropriation for Fiscal Year 2024; NIH’s Office of Laboratory Animal Welfare Suggests Flexibilities
NIH invites community feedback on upcoming efforts to develop and implement a set of minimum core common data elements for NIH-funded clinical research and trials.
NIH’s Office of AIDS Research will use its next Strategic Plan as a basis for developing the NIH HIV research budget, outlining HIV research priorities, and conveying information about NIH HIV research priorities to the scientific community.
The direct salary for individuals under NIH grant and cooperative agreement awards cannot exceed “Executive Level II” of the Federal Executive pay scale. Effective January 1, 2024, the salary limitation for Executive Level II is $221,900.
NIAID Is Operating Under a Continuing Resolution; Bookmark Helpful NIH SEED Resources for Small Businesses; Prepare Now for Opportunity on Cell and Gene Therapies for HIV Cure; Watch Webinar to Hone Applications for DEIA Supplements
The initiative is designed for early-stage investigators who want to initiate a research project in an area different from their previous research focus or training experience, and therefore have not produced preliminary data.
Prepare for Opportunity to Advance MCM Development for AMR Bacteria and Fungi; Submit Annual Reports to OLAW by December 1, 2023; Provide Feedback on HIV and Women’s Health, Consent Language for Digital Technologies.
An at-risk investigator has had substantial, independent NIH funding as a principal investigator and, unless successful in securing a substantial research grant award in the current fiscal year, will have no substantial research grant funding in the following fiscal year.
Be sure your Research Performance Progress Report encapsulates the most recent project period with a fresh summary. If your report text seems identical to last year, your program officer will worry there's been a lack of progress.
Stream Our Advisory Council Meeting on September 11 with NIH Videocast; Answer RFI on Dual Use Research of Concern and Potential Pandemic Pathogens Oversight; Provide Feedback on a Meaningful Adjustment to NIH’s Mission Statement; Help NIH Address Safety for Recombinant or Synthetic Nucleic Acid Molecules
The most recent analysis found a continual increase in the proportion of applications designating either female or underrepresented minority early-stage investigators both before and after the pandemic.
In which areas of biomedical research could novel alternative methods provide investigators with new means to answer key research questions in ways that cannot be accomplished currently through traditional methods like animal models?
A new resource from eRA describes red flags that users commonly encounter when using the Human Subjects System to report, view, and update a project’s human subjects enrollment, inclusion, and clinical trial data.
NIH’s Office of Research on Women’s Health offers training modules, videos, and other tools to instruct biomedical researchers on properly accounting for sex as a biological variable.
Share feedback on NIH's Herpes Simplex Virus Strategic Plan and its prioirities for basic research, diagnosis, treatment, cure, and prevention by June 21, 2023.
There are several types of concerns a researcher might need to report to NIH during the application process or while conducting NIH-supported research, from grant scams to research misconduct to peer-review integrity violations.
Be careful not to over-cite publications, such as preprints, as well as training- and resource-type awards. If an award’s only contribution to a publication is non-personnel resources, do not list the paper.
Federal income/excess profits taxes are strictly not allowable charges to NIH awards, either as direct or facilities and administrative costs. However, the small business fee may be used to pay for a tax liability.
NIH drafted a Public Access Plan that would alter the NIH Public Access Policy to reflect new priorities identified in a White House Office of Science and Technology Policy memorandum.
Start with NIH’s extensive guidance; a new page features sample data management and sharing plans and the frequently asked questions page explains how to account for data management and sharing in your budget.
To ensure a strong and diverse workforce and better understand workforce composition and participation in NIH programs, NIH regularly assesses the sex/gender, race, ethnicity,and disability status of its supported researchers.
HHS offers guidance on how to administer your program in compliance with applicable Civil Rights laws, e.g., sex discrimination and harassment, disability, and religious freedom.
Bookmark Updated Version of NIH Grants Policy Statement; Coming Soon: Reissued Parent NOFOs for Small Business Grant Applications; Review List of NIH Legislative Mandates for Fiscal Year 2024; Grants.gov Downtime Scheduled for this Weekend
Attend “NIH Grants Process: A Brief Walk-Through for Beginners” Event on May 15; Reminders for Small Business Concerns: TABA Supplements and Foreign Disclosures; Webinar to Discuss U54 Opportunity on Systems Modeling of Infection and Immunity
Attend Research Education Program Webinar on April 4, Apply by June 7; Public Access Policy Webpages Are on the Move; A Note on the Next NIH Grants Policy Statement Release
NIAID is hosting a virtual grant writing webinar series called “Debuting Your Research Career: How to Plan for and Write Your First (or Next) NIH Grant Application.” This monthly webinar series is free and open to pre- and post-doctoral fellows, clinician-scientists, and early-career investigators.
Simplified Review Criteria Will Receive a Soft Launch; Provide Input on HIV Research Priorities Before March 28, 2024; Attend Virtual Seminar on Opportunities for Early-Career Researchers; NIH Has a YouTube Channel for Grants Policy Discussions
Tune in for NIAID Advisory Council Meeting on January 30, 2024; Attend NIH Office of Extramural Research Webinar on Grants Policy Changes; eRA Commons ID Required for Senior/Key Personnel, Other Significant Contributors; Offer Feedback on the NIH Strategic Plan for Data Science
I-Corps Deadlines and Dates for FY 2024 Cohorts Announced; Materials from CHIVIM Clinical Studies and Trials Available; Small Business Collaboration with Resource-Limited Institutions.
Respond to a request for information on Potential Changes to the Policies for Oversight of Dual Use Research of Concern and the Potential Pandemic Pathogen Care and Oversight Policy Framework by October 16, 2023.
Dr. Jeanne Marrazzo Selected as NIAID’s Next Director; Collaborate with the AIDS Clinical Trials Group on HIV Remission and Cure; Stream Scientific Meeting on Congenital Cytomegalovirus Vaccine Research
The Cumulative Investigator Rate is calculated as the number of unique principal investigators (PIs) who were designated on an NIH research project grant divided by the number of unique PIs who were designated on applications over a 5-year period.
FDA and HHS’s Office for Human Research Protections published Draft Guidance to help stakeholders understand the processes used for review of research involving children as subjects that is not otherwise approvable by an IRB.
NIH supports many postdocs and graduate students through the Ruth L. Kirschstein National Research Service Award Program, which includes fellowship (“F”) and training (“T”) awards.
Attend Seminar on How Diversity Impacts Innovation in Team Science; New Visual Appearance Planned for Popular eRA Modules
NIH Natural Disaster Policy Applies to Recent Weather Events; Confirm Accuracy of Grants Information for Fiscal Year 2023; Share Feedback on Proposed Guidelines for Cephalopod Care and Use
NIH is considering revisions to the Public Access Policy following calls from the White House to enhance public access to the results of federally funded research. NIH’s Office of Science Policy will host a listening session on April 12, 2023.
NIAID is cohosting an Advances in Aging, Immunity, and Chronic Inflammatory Diseases workshop on May 9 and 10, 2023. The workshop will be hybrid, meaning there are options to attend in person or virtually.
A note for those conducting or planning COVID-19-related vaccine or treatment clinical trials and clinical studies supported by NIAID’s fiscal year 2020 emergency pandemic appropriations.
Advice Corner
Whether cultivating partnerships across fields and institutions resonates with your research goals, or you decide to pursue a notice of funding opportunity that requires collaborators, we suggest several resources to develop those pursuits.
The letters of support should reflect the roles and commitment of collaborators on the project as well as the rate and price for contracted services, while also aligning with your application’s research strategy and budget.
Learn how to better navigate the NIH grants application and award process.
Submitting complete and timely Federal Financial Reports is an important step to ensure NIAID’s prompt review and consideration of any carryover requests.
If you know your application will go to a Center for Scientific Review study section, you can request assignment to the most appropriate one.
The Small Business Administration (SBA) sets caps for total costs on small business grant awards for all federal agencies, above which an agency must request SBA approval.
NIAID recently posted two R01 sample applications and one K08 sample application to serve as examples of outstanding grantsmanship and demonstrate a successful approach to presenting a research plan.
If you or your institution receives a notification alerting you that a change was made to your application status, pay attention to the grant application number referenced in the message.
How familiar are you with NIAID’s solicitation and contract vocabulary? Test your knowledge by taking our quiz and pay attention to the explanations for each answer.
NIH requires disclosure of other support, foreign components, and conflicts of interest so that staff can ensure funded projects do not receive duplicative support, research is conducted objectively, and sensitive data are kept confidential.
When NIH issues a correction or update to an NIH Guide notice, the original notice’s text may not change since NIH can correct some fields in the original notice but not others.
Investigators must include plans for sharing unique model organisms and related resources in their grant applications or contract proposals. Investigators unable to share model organisms or resources are required to state why sharing is not feasible.
NIAID caps R01 research project grant renewals at 20 percent above the direct costs of the last competing award period, less the costs of certain items like subawards’ facilities and adminsitrative costs.
As an applicant or a funded investigator, there's a good chance you'll need guidance on administrative or budget matters related to your grant or cooperative agreement.
An ancillary study is an independent research project that uses samples or data from a parent study to extend knowledge in scientific areas beyond the original scope of the parent study. It may qualify as a clinical study or a clinical trial.
Most notices of funding opportunities list the application due date, peer review date, Advisory Council round, and earliest project start date in the “Key Dates” section of Part 1. Overview Information.
If you identify a mistake or a missing form while examining your application in the viewing window, then your signing official should reject the application so that you can correct the error and submit a replacement application.
NIH uses notices of special interest (NOSIs) to invite applications on high-priority and high-opportunity areas of science. NOSIs are designed to reduce administrative burden on NIH staff and grant applicants alike.
NIH staff use award citations to evaluate the impact of grant funding on scientific progress over time. Similarly, peer reviewers rely on award citations to help assess award productivity.
A competing revision provides additional funding for activities that call for an expansion of your original scope of award, whereas administrative supplements fund activities that, while unforeseen when the application was being submitted, are still within the scope of the project.
NIH’s Office of Research Infrastructure Programs supports purchases of state-of-the-art, commercially available instruments on shared-use basis to enable or enhance conduct of cutting-edge research by NIH-funded investigators.
Try to incorporate new preliminary data, literature citations, letters of reference, and any other relevant updates that came about in the months between submissions.
NIH defines a clinical trial as a research study in which one or more human subjects are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effects of those interventions on health-related biomedical or behavioral outcomes.
Any recipient of federal support and the information they submit to the government is subject to the Freedom of Information Act (FOIA).
Within eRA’s Prior Approval Module, a variety of templates exist to facilitate the most common request types: carryover, change of investigator, no-cost extension, big grants, and withdrawal.
If you are planning a delayed start to a human subjects study but wrongly mark it as a delayed onset study, your application will be missing important details which may negatively affect your score.
Even successful partnerships experience disagreements and disputes. To ensure those moments don’t derail your science, you need to have an established conflict resolution plan.
Whom should you ask for help with general matters like identifying an appropriate funding opportunity or discovering available resources?
Any time you read an NIAID notice of funding opportunity, build a habit of checking whether there is an associated questions and answers page.
Overlap may be scientific, budgetary, or commitment related. To resolve concerns around overlap, you’ll need to provide information as part of the Just-in-Time (JIT) process that occurs after peer review, when NIAID is considering the application for funding.
You may ask your institutional business office for preaward spending approval to cover costs up to 90 days before the start date of the initial budget period of a new or renewal award.
The NIH inclusion policy ensures that children must be included in all NIH-conducted human subjects research unless there are scientific or ethical reasons for exclusion.
Request a budget sufficient to make your proposed project successful. Do not risk peer reviewers judging your costs insufficient to the work or deeming you naive to the price of conducting successful research.
Our career development award programs support postdoctoral scholars and scientists at early- and mid-career stages, including those from diverse backgrounds or returning to biomedical careers.
To be eligible, you must be a doctoral-level clinician or researcher who is committed to conducting NIH-relevant research at a domestic, non-profit, or government organization.
When you’re ready to publicize your scientific discoveries, let NIAID help spread the word. Through a variety of communication vehicles, our News and Science Writing Branch highlights the work of NIAID-funded researchers.
While many NIAID careers require expertise specific to our area of science, other positions don’t require an advanced or scientific degree.
NIH grant recipients have 120 days from the end of a performance period to liquidate obligations by drawing funds from the Payment Management System.
We cover role-related terms that have caused confusion like co-investigator, collaborator, consultant, other significant contributor, and staff scientist.
Collaborators may use different types of agreements to facilitate partnerships, including research collaboration agreements, material transfer agreements, and data transfer agreements.
Know how to respond if you get the error message “Data Management and Sharing Costs can only be entered on one of the lines in Section F Other Direct Costs within the same budget period."
In practice, most mentees need a developmental mentoring network that consists of multiple social relationships each fostering career development and personal growth.
Foreign applications need to propose talent, resources, populations, or environmental conditions not in the United States, e.g., access to a unique study population.
In order to spend unobligated funds from an earlier budget period during a no-cost extension, absent automatic carryover authority, you first need to confirm NIAID’s prior approval of a carryover.
The eligibility of a principal investigator differs from that of an institution. This is important in the context of fellowships and career development awards, where non-citizens may be working at U.S. institutions, or U.S. citizens may be working at international institutions.
NIAID strongly encourages consultations to apprise principal investigators of the time, cost, and regulatory obligations that come with seeking and receiving an investigator-initiated clinical trial award.
NIH expects researchers to use unbiased and well-controlled experimental design, methodology, analysis, interpretation, and reporting of results.
Three new high-scoring, successful G11 samples reflect excellent grantsmanship and serve as educational resources for other applicants and award administrators.
NIAID cannot correct an error spotted in your application after the receipt deadline on your behalf, even if an overlooked content error would render your application nonresponsive to an initiative's requirements.
Biosafety in Microbiological and Biomedical Laboratories is an advisory document recommending best practices for safely conducting work in biomedical and clinical laboratories from a biosafety perspective.
Reader Questions
Contact Us
Email us at deaweb@niaid.nih.gov for help navigating NIAID’s grant and contract policies and procedures.

